Pneumococcal Vaccination: What Everyone Should Know
One of the Recommended Vaccines by Disease
Key Facts
Pneumococcal disease is common in young children, but older adults are at greatest risk of serious pneumococcal infections and even death. CDC recommends vaccination with the pneumococcal conjugate vaccine for all babies and children younger than 2 years old, all adults 65 years or older, and people 2 years through 64 years old who are at increased risk for pneumococcal disease due to certain medical conditions.
Who Should Get Pneumococcal Vaccines?
CDC recommends pneumococcal vaccination for all babies and children younger than 2 years old and all adults 65 years or older. In certain situations, other children and adults should also get pneumococcal vaccines. Below is more information about who should and should not get each type of pneumococcal vaccine.
Talk to your or your child’s healthcare professional about what is best for your specific situation.
Learn about different types and brands of pneumococcal vaccines at What Types of Pneumococcal Vaccines Are there?
Pneumococcal Conjugate Vaccine
CDC recommends vaccination with the pneumococcal conjugate vaccine (PCV13 or Prevnar 13®) for
- All babies and children younger than 2 years old
- All adults 65 years or older
- People 2 through 64 years old who are at increased risk for disease due to certain medical conditions
Pneumococcal Polysaccharide Vaccine
CDC recommends vaccination with the pneumococcal polysaccharide vaccine (PPSV23 or Pneumovax23®) for
- All adults 65 years or older
- People 2 through 64 years old who are at increased risk for disease due to certain medical conditions
- Adults 19 through 64 years old who smoke cigarettes
Who Should Not Get These Vaccines?
Because of age or health conditions, some people should not get certain vaccines or should wait before getting them. Read the guidelines below and ask your or your child’s healthcare professional for more information.
Pneumococcal Conjugate Vaccine
Tell the person who is giving you or your child a pneumococcal conjugate vaccine if:
You or your child have had a life-threatening allergic reaction or have a severe allergy.
- Anyone who has had a life-threatening allergic reaction to a dose of this vaccine, to an earlier pneumococcal conjugate vaccine called PCV7 (or Prevnar®), or to any vaccine containing diphtheria toxoid (for example, DTaP) should not get PCV13.
- Anyone with a severe allergy to any component of PCV13 should not get the vaccine. Your or your child’s healthcare professional can tell you about each vaccine’s ingredients.
You or your child are not feeling well.
- People who have a mild illness, such as a cold, can probably get the vaccine. People who are moderately or severely ill should probably wait until they recover. Your or your child’s healthcare professional can advise you.
You are pregnant.
- There is no evidence on the safety of PCV13 use during pregnancy. As a precaution, women who need the vaccine should be vaccinated before becoming pregnant, if possible.
Pneumococcal Polysaccharide Vaccine
Children younger than 2 years old should not get this vaccine. In addition, tell the person who is giving you or your child a pneumococcal polysaccharide vaccine if:
You or your child have had a life-threatening allergic reaction or have a severe allergy.
- Anyone who has had a life-threatening allergic reaction to PPSV23 should not get another dose.
- Anyone who has a severe allergy to any component of PPSV23 should not get it. Your or your child’s healthcare professional can tell you about each vaccine’s ingredients.
You or your child are not feeling well.
- People who have a mild illness, such as a cold, can probably get the vaccine. People who are moderately or severely ill should probably wait until they recover. Your or your child’s healthcare professional can advise you.
You are pregnant.
- There is no evidence that PPSV23 is harmful to either a pregnant woman or to her baby. However, as a precaution, women who need the vaccine should be vaccinated before becoming pregnant, if possible.
What Types of Pneumococcal Vaccines Are There?
There are two pneumococcal vaccines that are licensed for use in the United States by the Food and Drug Administration (FDA):
- Pneumococcal conjugate vaccine (PCV13 or Prevnar 13®)
- Pneumococcal polysaccharide vaccine (PPSV23 or Pneumovax23®)
Helpful Terms
- Conjugate: A type of vaccine that joins a protein to part of the bacteria to improve the protection the vaccine provides
- Polysaccharide: A type of vaccine that is made to look like the surface of certain bacteria in order to help the body build protection against that germ
Pneumococcal Conjugate Vaccine
- Prevnar 13® [42 pages]: This vaccine is given in a three-dose primary series starting at 2 months of age plus one booster dose at 12 through 15 months of age. Children who begin vaccination after 6 months of age will receive fewer doses. Adults who are recommended to receive it only need a single dose. The vaccine helps protect against the 13 types of pneumococcal bacteria that are the most common causes of serious infections in children and adults. It can also help prevent some ear infections.
Pneumococcal Polysaccharide Vaccine
- Pneumovax23® [9 pages]: This vaccine is given as a single dose to people who are recommended to receive it. One or two booster doses are recommended for some people. This vaccine helps protect against 23 types of pneumococcal bacteria.
How Well Do These Vaccines Work?
Summary
Some pneumococcal infections are considered “invasive.” Invasive disease means that germs invade parts of the body that are normally free from germs. Invasive disease is usually very serious and can sometimes result in death.
Vaccines that help protect against pneumococcal disease work well, but cannot prevent all cases.
Studies* show that at least 1 dose of pneumococcal conjugate vaccine (PCV13 or Prevnar 13®) protects
- At least 8 out of 10 babies from invasive pneumococcal disease
- 75 out of 100 adults 65 years or older against invasive pneumococcal disease
- 45 out of 100 adults 65 years or older against pneumococcal pneumonia
Studies* show that 1 dose of pneumococcal polysaccharide vaccine (PPSV23 or Pneumovax23®) protects
- Between 50 to 85 out of 100 healthy adults against invasive pneumococcal disease
In Depth
Studies done on the first pneumococcal conjugate vaccine (PCV7 or Prevnar 7®), which was licensed by the Food and Drug Administration (FDA) in 2000, showed the vaccine is highly effective in preventing invasive pneumococcal disease in young children. PCV13, which was licensed by FDA in 2010, is similar to PCV7 but provides protection against infections caused by 6 more types (serotypes) of pneumococcal bacteria that can cause disease. Studies show that PCV13 causes the body’s immune system to create protective antibodies, which help fight the pneumococcal bacteria, similar to PCV7.
In a study including 37,000 babies in California, PCV7 protected more than 9 out of every 10 babies from invasive disease caused by serotypes included in the vaccine. The children who got the vaccine also had fewer ear infections (otitis media) and fewer ear tubes (tympanostomy tubes) placed. The vaccine was also shown to prevent pneumonia in children.
CDC conducted a study soon after PCV7 was licensed and found that the vaccine protected nearly all (96%) healthy children against pneumococcal disease caused by the 7 serotypes in the vaccine. In addition, 8 out of 10 (81%) children with medical conditions that put them at increased risk of pneumococcal disease were protected after receiving at least one dose of PCV7. The vaccine was also highly effective at preventing pneumococcal disease caused by serotypes included in the vaccine that are resistant to medications used to treat bacterial infections (antibiotics).
Studies conducted a few years after PCV13 was licensed showed that at least one dose of PCV13 protects 8 out of every 10 babies from invasive disease caused by serotypes included in the vaccine, and this protection was similar among children with and without medical conditions that put them at increased risk of pneumococcal disease. The vaccine is also effective at preventing pneumococcal disease caused by serotypes included in the vaccine that are resistant to antibiotics.
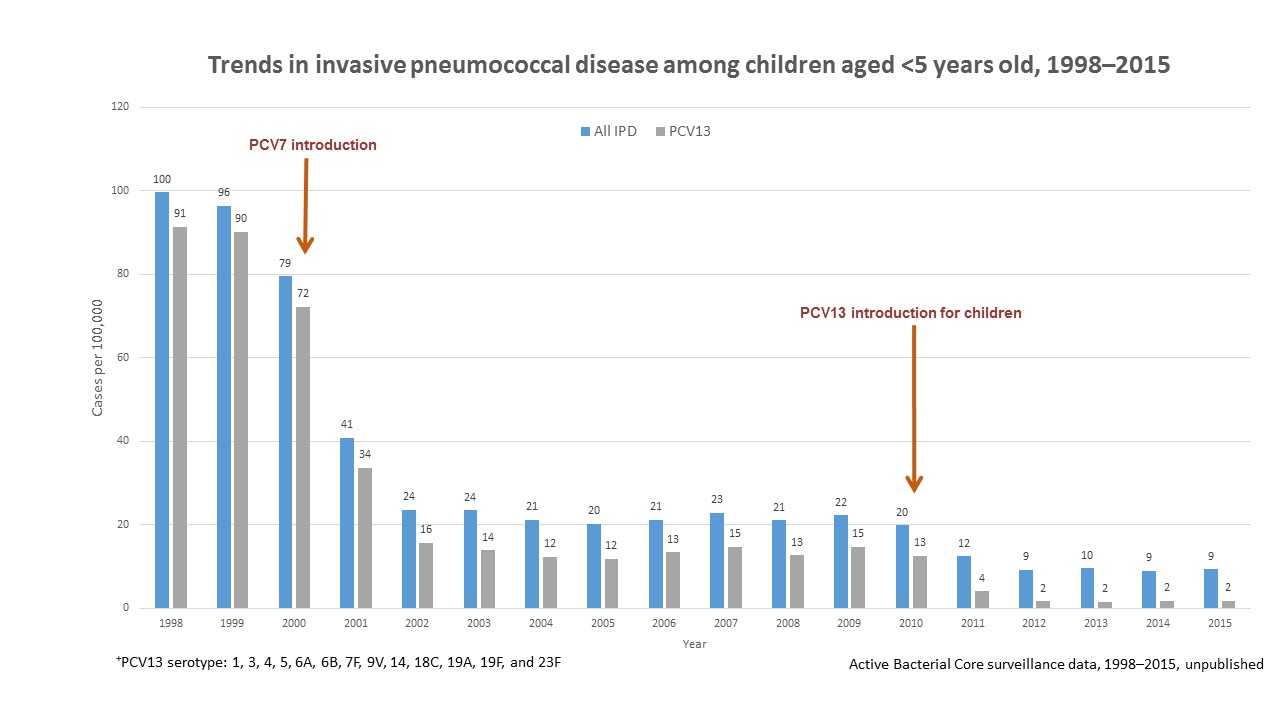
View Larger
The United States saw large drops in rates of invasive pneumococcal disease caused by serotypes included in pneumococcal vaccines (PCV7 and PCV13) after routine use of the vaccines began for children.
Since routine use of PCV7 for children began in the United States, rates of invasive pneumococcal disease caused by the seven serotypes included in the vaccine have declined by 99%. Decreases in disease rates have been seen among unvaccinated people of all ages, including babies too young to be vaccinated and older adults. This indicates that vaccine use has reduced transmission (spread) of strains that cause disease from vaccinated children to those who are unvaccinated. Rates of invasive pneumococcal disease caused by some serotypes not in PCV7 have increased since then. However, these increases have been small compared to the decreases in serotypes included in the vaccine. Also, the main serotypes causing the increases after PCV7 are covered by PCV13, and the rates of disease caused by these serotypes have declined since PCV13 replaced PCV7 for routine use among children. It is estimated that more than 30,000 cases of invasive pneumococcal disease and 3,000 deaths were prevented in the first 3 years of using PCV13.
In 2011, FDA licensed PCV13 for use among adults 50 years or older. In a study including approximately 85,000 adults 65 years or older in the Netherlands, PCV13 protected 75 out of 100 of those vaccinated against invasive pneumococcal disease and 45 out of 100 vaccinated against pneumococcal pneumonia caused by the serotypes included in the vaccine.
PPSV23 protects between 50 to 85 out of every 100 adults with healthy immune systems against invasive disease caused by the 23 serotypes covered by the vaccine.
* Studies looked at protection against infections caused by the serotypes covered by the specific vaccine used.
What Are the Possible Side Effects-Pneumococcal Vaccines?
Most people who get a pneumococcal vaccine do not have any serious problems with it. With any medicine, including vaccines, there is a chance of side effects. These are usually mild and go away on their own within a few days, but serious reactions are also possible.
Mild Problems
Pneumococcal Conjugate Vaccine
Mild problems following pneumococcal conjugate vaccination can include:
- Reactions where the shot was given
- Redness
- Swelling
- Pain or tenderness
- Fever
- Loss of appetite
- Fussiness (irritability)
- Feeling tired
- Headache
- Chills
Young children who get pneumococcal conjugate vaccination along with inactivated flu vaccine at the same time may be at increased risk for seizures caused by fever. Ask your doctor for more information.
Pneumococcal Polysaccharide Vaccine
Mild problems following pneumococcal polysaccharide vaccination can include:
- Reactions where the shot was given
- Redness
- Pain
- Fever
- Muscle aches
If these problems occur, they usually go away within about two days.
Problems that Could Happen After Getting Any Injected Vaccine
- People sometimes faint after a medical procedure, including vaccination. Sitting or lying down for about 15 minutes can help prevent fainting and injuries caused by a fall. Tell your healthcare professional if you or your child feels dizzy, have vision changes, or have ringing in the ears.
- Some people get severe pain in the shoulder and have difficulty moving the arm where a shot was given. This happens very rarely.
- Any medicine can cause a severe allergic reaction. Such reactions from a vaccine are very rare, estimated at about 1 in a million doses, and would happen within a few minutes to a few hours after the vaccination.
- As with any medicine, there is a very remote chance of a vaccine causing a serious injury or death.
For more information on possible side effects from vaccination, visit CDC’s Possible Side-effects from Vaccines webpage.
Where Can I Find These Vaccines?
Your healthcare professional’s office is usually the best place to receive recommended vaccines for you or your child.
These vaccines are part of the routine childhood immunization schedules. Therefore, vaccines are regularly available at pediatric and family practice offices, as well as community health clinics and public health departments for children.
If your healthcare professional does not have pneumococcal vaccines for adults, ask for a referral.
Pneumococcal vaccines may also be available at pharmacies, workplaces, community health clinics, health departments, or other community locations such as schools and religious centers. Federally funded health centers can also provide services if you don’t have a regular source of health care. Locate one near you. You can also contact your state health department to learn more about where to get pneumococcal vaccines in your community.
When receiving any vaccine, ask the provider to record the vaccine in the state or local registry, if available. This helps healthcare professionals at future encounters know what vaccines you or your child have already received.
How Do I Pay for These Vaccines?
There are several ways pneumococcal vaccines may be paid for:
Medicare
Medicare Part B covers 100% of the cost for both pneumococcal vaccines (when administered at least 12 months apart).
Private Health Insurance
Most private health insurance plans cover pneumococcal vaccines. Check with your insurance provider for details on whether there is any cost to you and for a list of in-network vaccine providers.
Vaccines for Children Program
The Vaccines for Children (VFC) Program provides vaccines to children whose parents or guardians may not be able to afford them. A child is eligible for the program if they are younger than 19 years of age and meet one of the following requirements:
- Medicaid-eligible
- Uninsured
- American Indian or Alaska Native
- Underinsured (have health insurance that does not cover vaccines or does not cover certain vaccines)
If your child is VFC-eligible, ask if your healthcare professional is a VFC provider. For help in finding a VFC provider near you, contact your state or local health department’s VFC Program Coordinator or call CDC at 1-800-CDC-INFO (232-4636).
References
- Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–25.
- Moore MR, Link-Gelles R, Schaffner W, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: A matched case-control study. Lancet Respir Med. 2016 Mar 14. [ePub ahead of print]
- Pilishvili T, Bennett NM. Pneumococcal disease prevention among adults: Strategies for the use of pneumococcal vaccines. Vaccine. 2015;33(4):D60–5.
Related Pages
- CDC’s Pneumococcal Disease Website
- Educational Materials on Pneumococcal Disease
- Easy-to-Read Schedules
- Pneumococcal Vaccine Information Statements
- Pneumococcal Conjugate (PCV13) (English / Other Languages)
- Pneumococcal Polysaccharide (PPSV23) (English / Other Languages)
- Vaccine Safety
- CDC’s Vaccine Safety Website
-
Safety and Immunogenicity of a 13-Valent Pneumococcal Conjugate Vaccine
PEDIATRICS Vol. 125 No. 5 May 2010, pp. 866-875 Posted May 2010 - Pneumococcal Vaccine Safety Website: A Closer Look at the Safety Data
- Frequently Asked Questions about Vaccine Safety
- State Mandates
- Vaccines for Children Program
- Information for the General Public: Cochlear Implants and Vaccination Recommendations
- Page last reviewed: November 22, 2016
- Page last updated: September 13, 2017
- Content source:


 ShareCompartir
ShareCompartir