US Medical Eligibility Criteria (US MEC) for Contraceptive Use
US Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2016
The United States Medical Eligibility Criteria for Contraceptive Use, 2016 (US MEC) includes recommendations for using specific contraceptive methods by women and men who have certain characteristics or medical conditions. The recommendations in this report are intended to assist health care providers when they counsel women, men, and couples about contraceptive method choice. Notable updates include the addition of recommendations for women with cystic fibrosis, women with multiple sclerosis, and women using certain psychotropic drugs or St. John’s wort; revisions to the recommendations for emergency contraception, including the addition of ulipristal acetate; and revisions to the recommendations for postpartum women, women who are breastfeeding, and women with known dyslipidemias, migraine headaches, superficial venous disease, gestational trophoblastic disease, sexually transmitted diseases, human immunodeficiency virus, or who are using antiretroviral therapy.
These recommendations for health care providers were updated by CDC after review of the scientific evidence and consultation with national experts who met in Atlanta, Georgia, during August 26–28, 2015. The information in this report updates the 2010 U.S. MEC (CDC. U.S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR 2010:59 [No. RR-4]). Although these recommendations are meant to serve as a source of clinical guidance, health care providers should always consider the individual clinical circumstances of each person seeking family planning services. This report is not intended to be a substitute for professional medical advice for individual patients. Persons should seek advice from their health care providers when considering family planning options.
Downloads and Resources

Print Version of the MMWR MEC Update [PDF – 94KB]
Revised recommendations for the Use of Hormonal Contraception Among Women at High Risk for HIV Infection

U.S. MEC Speaker Ready Slide Set
This slide presentation can be used by health care providers who want to increase knowledge about the US MEC.. The presentation and speaker notes can also be used or adapted to give presentations on the US MEC. This material is in the public domain and may be used and reprinted without permission; citation as to source, however, is appreciated. Powerpoint presentation available in ppt or pdf formats. If you would like to received continuing education credits, visit the training page.

Teen Pregnancy Prevention Speaker Ready Slide Set
This slide presentation can be used by health care providers who want to increase knowledge about the U.S. MEC, U.S. SPR, and Teen Pregnancy Prevention. The presentation and speaker notes can also be used or adapted to give presentations on the U.S. MEC, U.S. SPR, and Teen Pregnancy Prevention. This material is in the public domain and may be used and reprinted without permission; citation as to source, however, is appreciated. Powerpoint presentation available in ppt or pdf formats. If you would like to received continuing education credits, visit the training page.
[PPT – 14.4MB] | [PDF – 5.51MB]
US MEC & US SPR App
Download the 2016 US MEC and US SPR app, an easy to use reference that combines information from the both CDC family planning guidance. It features a streamlined interface so providers can access the guidance quickly and easily. It is available for iOS and Android operating systems.
iOS (Apple Store) App
Android (Google Play Store) App

Print Version of the MMWR [PDF – 1.82MB]
US Medical Eligibility Criteria for Contraceptive Use, 2016

MEC Summary Chart (English) [PDF – 323 KB] | MEC Summary Chart (Spanish) [PDF – 443 KB]
8 ½ by 14 charts can be printed double sided, laminated, and used by health care providers when counseling women.
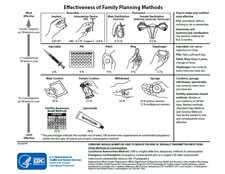
Effectiveness of Contraceptive Methods Chart [PDF – 670KB]
Charts can be printed double sided, laminated, and used by health care providers when counseling women
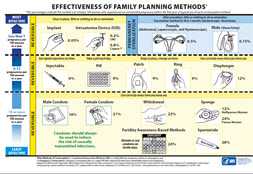
Effectiveness of Family Planning Methods [PDF – 305KB] (in English)
Eficacia de los métodos de planificación familiar [PDF – 136 KB] (in Spanish)
2’ x 3’ Posters can be printed double sided, laminated, and used by health care providers when counseling women.

MEC Wheel [PDF – 63 KB]
The MEC Wheel, other provider tools and MMWRs are available to order from CDC-INFO on Demand in limited quantities.
- Page last reviewed: September 26, 2017
- Page last updated: September 26, 2017
- Content source:


 ShareCompartir
ShareCompartir