Surveillance and Reporting
Doctors consider some pneumococcal infections to be “invasive.” Invasive disease means that germs invade parts of the body that are normally free from germs. Invasive pneumococcal disease includes infections of the tissue covering the brain and spinal cord (meningitis) and bloodstream (bacteremia).
Trends
Following the introduction of the pneumococcal conjugate vaccines in the United States (PCV7 in 2000 and PCV13 in 2010), CDC reported dramatic declines in invasive pneumococcal disease among children less than 5 years old (See Figure 1). Overall, invasive pneumococcal disease decreased from 100 cases per 100,000 people in 1998 to 9 cases per 100,000 in 2015. Invasive pneumococcal disease caused by the 13 serotypes covered by PCV13 decreased from 91 cases per 100,000 people in 1998 to 2 cases per 100,000 people in 2015.
Figure 1.
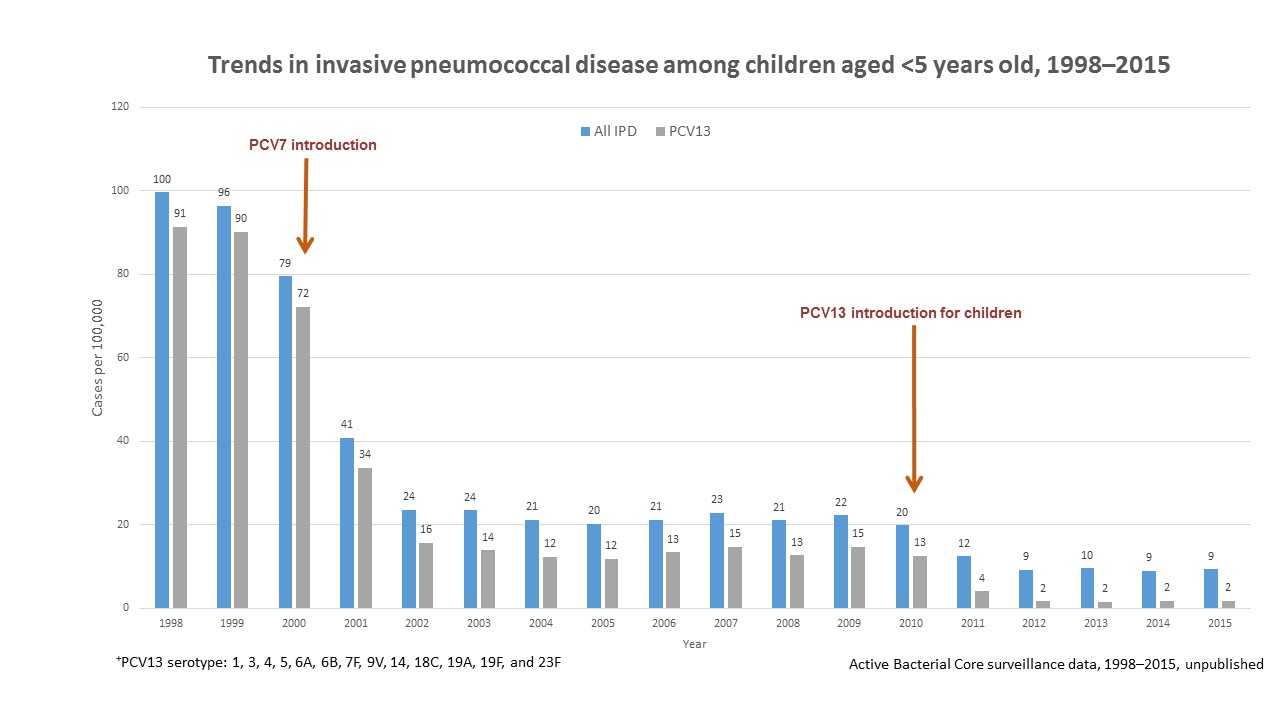
Figure 1 shows changes in the incidence of invasive pneumococcal disease (IPD) among children <5 years old from 1998 through 2015 in the United States. Rates of IPD expressed as cases per 100,000 population are shown on the y-axis, and calendar year of surveillance on the x-axis. Blue bars represent overall IPD incidence, while the grey bars represent IPD incidence caused by serotypes included in the 13-valent pneumococcal conjugate vaccine (PCV13). Pneumococcal 7-valent conjugate vaccine (PCV7), containing serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, was introduced for use among children <5 years old in 2000. PCV13, containing serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F, was introduced for use among children <5 years old in 2010. The overall IPD incidence declined from 100 cases per 100,000 in 1998 to 9 cases per 100,000 in 2015; IPD caused by PCV13 serotypes declined from 91 cases per 100,000 in 1998 to 2 cases per 100,000 in 2015.
PCV13 was introduced in 2012 for use among adults 19 years or older with immunocompromising conditions and in 2014 for all adults 65 years or older. However, declines in invasive pneumococcal disease were seen as early as 2001 among adults between the ages of 19 and 64 years old (see Figure 2 below) and adults 65 years or older (see Figure 3 below) because of the use of pneumococcal conjugate vaccines in children (herd protection). Overall, invasive pneumococcal disease in adults 19 through 64 years old decreased from 16 cases per 100,000 people in 1998 to 7 cases per 100,000 people in 2015. Invasive pneumococcal disease caused by the 13 serotypes included in PCV13 decreased in adults 19 through 64 years old from 11 cases per 100,000 in 1998 to 2 cases per 100,000 people in 2015.
Overall, invasive pneumococcal disease in adults 65 or older decreased from 59 cases per 100,000 people in 1998 to 23 cases per 100,000 people in 2015. Invasive pneumococcal disease caused by the 13 serotypes included in PCV13 in adults 65 or older decreased from 44 cases per 100,000 in 1998 to 5 cases per 100,000 people in 2015.
Of note, invasive pneumococcal disease caused by the serotypes covered by pneumococcal 23-valent polysaccharide vaccine (PPSV23) also declined from 14 cases per 100,000 people in 1998 to 5 cases per 100,000 people in 2015 in adults 19 to 64 years old, and from 51 cases per 100,000 people in 1998 to 13 cases per 100,000 people in 2015 in adults 65 or older. However, these reductions were due to declines in invasive pneumococcal disease caused by serotypes in common with PCV13. PPSV23 has been available since 1984 and is recommended for all adults 65 years of age or older and for persons 2 years or older with chronic medical conditions.
Figure 2.
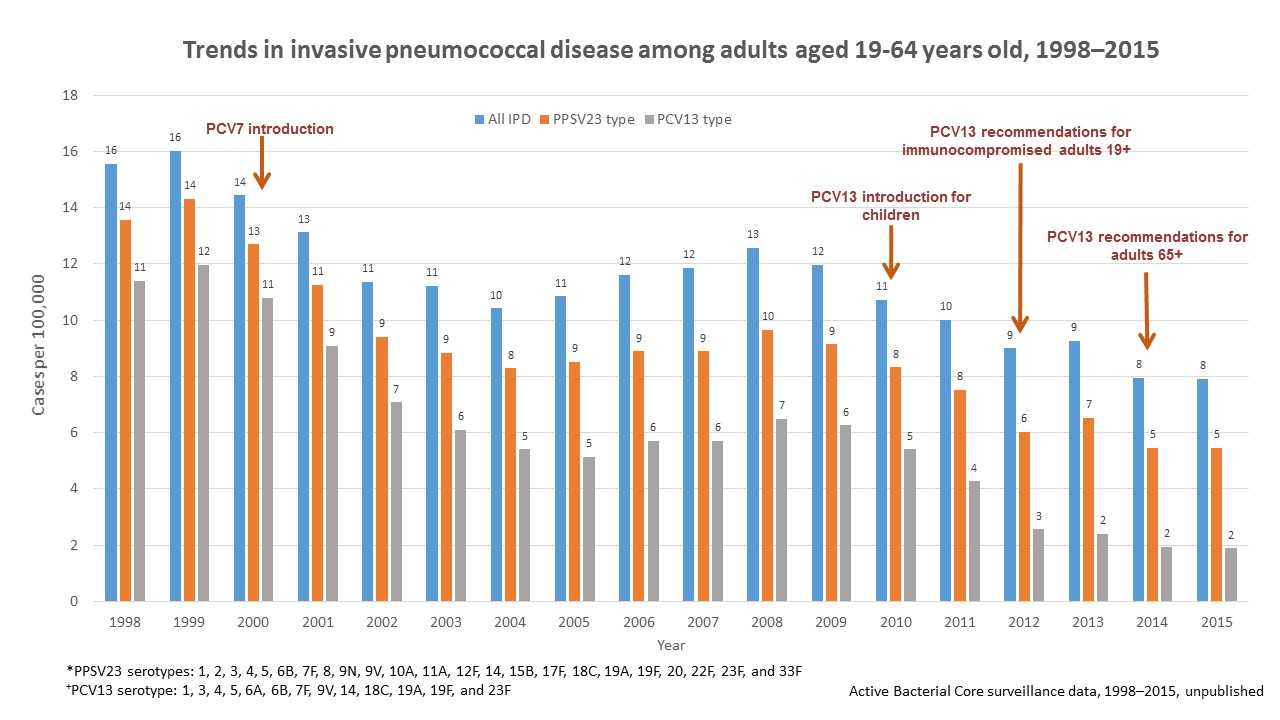
Figure 2 shows changes in the incidence of invasive pneumococcal disease (IPD) among adults 19 through 64 years of age from 1998 through 2015 in the Unites States. Rates of IPD expressed as cases per 100,000 population are shown on the y-axis, and calendar year of surveillance on the x-axis. Blue bars represent overall IPD incidence, orange bars represent IPD incidence caused by serotypes included in the 23-valent pneumococcal polysaccharide vaccine (PPSV23), while the grey bars represent IPD incidence caused by serotypes included in the 13-valent pneumococcal conjugate vaccine. PPSV23, containing serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F, has been available since 1984 and recommended for all adults 65 years or older and for people 2 years or older with chronic medical conditions. Pneumococcal 7-valent conjugate vaccine (PCV7), containing serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, was introduced for use among children <5 years old in 2000. Pneumococcal 13-valent conjugate vaccine (PCV13) containing serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F, was introduced for use among children <5 years old in 2010, for adults 19 years or older with immunocompromising conditions in 2012, and for all adults 65 years or older in 2014. The overall IPD incidence declined from 16 cases per 100,000 in 1998 to 7 cases per 100,000 in 2015; IPD caused by PCV13 serotypes declined from 11 cases per 100,000 in 1998 to 2 cases per 100,000 in 2015. IPD caused by PPSV23 serotypes also declined from 14 cases per 100,000 in 1998 to 5 cases per 100,000 in 2015, but these reductions were due to declines in IPD caused by serotypes in common with PCV13.
Figure 3.
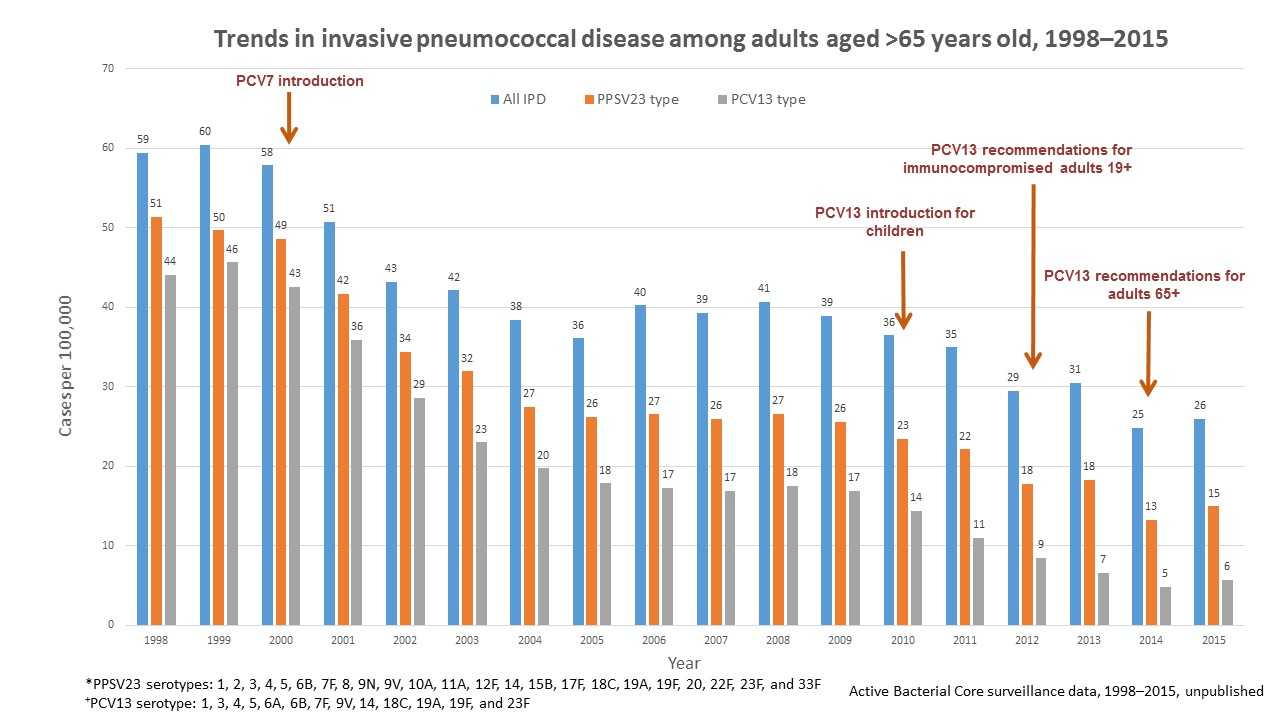
Figure 3 shows changes in the incidence of invasive pneumococcal disease (IPD) among adults 65 years or older from 1998 through 2015 in the Unites States. Rates of IPD expressed as cases per 100,000 population are shown on the y-axis, and calendar year of surveillance on the x-axis. Blue bars represent overall IPD incidence, orange bars represent IPD incidence caused by serotypes included in 23-valent pneumococcal polysaccharide vaccine (PPSV23), while the grey bars represent IPD incidence caused by serotypes included in the 13-valent pneumococcal conjugate vaccine (PCV13). PPSV23, containing serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F, has been available since 1984 and recommended for all adults 65 years or older and for people 2 years or older with chronic medical conditions. Pneumococcal 7-valent conjugate vaccine (PCV7), containing serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, was introduced for use among children <5 years old in 2000. Pneumococcal 13-valent conjugate vaccine (PCV13), containing serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F, was introduced for use among children <5 years old in 2010, for adults 19 years older with immunocompromising conditions in 2012, and for all adults 65 years or older in 2014. The overall IPD incidence declined from 59 cases per 100,000 in 1998 to 23 cases per 100,000 in 2015; IPD caused by PCV13 serotypes declined from 44 cases per 100,000 in 1998 to 5 cases per 100,000 in 2015. IPD caused by PPSV23 serotypes also declined from 51 cases per 100,000 in 1998 to 13 cases per 100,000 in 2015, but these reductions were due to declines in IPD caused by serotypes in common with PCV13.
Surveillance
Invasive pneumococcal disease is nationally notifiable meaning the laboratory who identifies the bacteria or the healthcare provider who is caring for the patient must report the case to the appropriate health department. State health departments then report invasive pneumococcal disease cases to CDC through the National Notifiable Diseases Surveillance System (NNDSS). Some regions in the United States also use Active Bacterial Core surveillance (ABCs), a core component of CDC’s Emerging Infections Programs (EIP) network, to report cases. State health departments do not report cases of non-invasive pneumococcal disease, like ear and sinus infections, through either surveillance system.
National Notifiable Diseases Surveillance System
State health departments report invasive pneumococcal disease to CDC through NNDSS. Health departments should report both suspected and confirmed cases nationally.
Active Bacterial Core surveillance
In addition to reporting through the state health department, some regions in the United States also track invasive pneumococcal disease cases through ABCs. ABCs is an active, laboratory- and population-based surveillance system for invasive bacterial pathogens of public health importance. Researchers and public health professionals have used ABCs data to track disease trends, including the decline in pneumococcal disease following the introduction of the pediatric pneumococcal conjugate vaccine.
Related Links
-
2009 Position Statement from the Council of State and Territorial Epidemiologists (CSTE) [7 pages]
CSTE’s 2009 position statement focuses on enhancing state-based surveillance for invasive pneumococcal disease.
- Page last reviewed: September 6, 2017
- Page last updated: September 6, 2017
- Content source:


 ShareCompartir
ShareCompartir