Meeting the Healthy People 2020 Objectives to Reduce Cancer Mortality
ORIGINAL RESEARCH — Volume 12 — July 2, 2015
Hannah K. Weir, PhD; Trevor D. Thompson, BS; Ashwini Soman, MBBS, MPH; Bjorn Møller, PhD; Steven Leadbetter, MS; Mary C. White, ScD
Suggested citation for this article: Weir HK, Thompson TD, Soman A, Møller B, Leadbetter S, White MC. Meeting the Healthy People 2020 Objectives to Reduce Cancer Mortality. Prev Chronic Dis 2015;12:140482. DOI: http://dx.doi.org/10.5888/pcd12.140482.
PEER REVIEWED
PEER REVIEWED
Abstract
Introduction
Healthy People 2020 (HP2020) calls for a 10% to 15% reduction in death rates from 2007 to 2020 for selected cancers. Trends in death rates can be used to predict progress toward meeting HP2020 targets.
Methods
We used mortality data from 1975 through 2009 and population estimates and projections to predict deaths for all cancers and the top 23 cancers among men and women by race. We apportioned changes in deaths from population risk and population growth and aging.
Results
From 1975 to 2009, the number of cancer deaths increased among white and black Americans primarily because of an aging white population and a growing black population. Overall, age-standardized cancer death rates (risk) declined in all groups. From 2007 to 2020, rates are predicted to continue to decrease while counts of deaths are predicted to increase among men (15%) and stabilize among women (increase <10%). Declining death rates are predicted to meet HP2020 targets for cancers of the female breast, lung and bronchus, cervix and uterus, colon and rectum, oral cavity and pharynx, and prostate, but not for melanoma.
Conclusion
Cancer deaths among women overall are predicted to increase by less than 10%, because of, in part, declines in breast, cervical, and colorectal cancer deaths among white women. Increased efforts to promote cancer prevention and improve survival are needed to counter the impact of a growing and aging population on the cancer burden and to meet melanoma target death rates.
Introduction
In the United States, the age-standardized cancer death rate began declining in the early 1990s, largely because of declines in deaths from lung and prostate cancer in men, breast cancer in women, and colorectal cancer in both sexes (1). The age-standardized death rate approximates the population’s risk of dying from cancer and is used to compare risk of death between populations or over time within a population. A decline in the death rate means that the overall risk of dying from cancer in the population has decreased. However, age-standardized rates do not convey the full extent of the cancer burden, as they effectively remove the influence of demographic changes in the population. During this time, the observed number of cancer deaths has continued to increase (2).
The number of cancer deaths is a function of the population’s risk of dying from cancer and the population’s age structure and size. The observed increase in the number of cancer deaths reflects the increased risk of dying from cancer with age, and during the past several decades, the US population has grown, particularly in the older age groups (3). These demographic trends and increasing cancer burden are forecast to continue as the cohort born following World War II enters the age groups most at risk of dying from cancer (4).
In 1971, the US Congress passed the National Cancer Act, which signaled a national effort against cancer (5) and led to the establishment of the Surveillance, Epidemiology, and End Results (SEER) Program in 5 states and 4 metropolitan areas (1). Since then, cancer registries (2) and cancer control programs (6) have been established in all states. More recently, the US Department of Health and Human Services (HHS) issued Healthy People 2020 (HP2020) (7), which included several objectives for reducing cancer mortality. Each objective has a baseline measure and a target to be achieved by 2020. Most of the cancer mortality objectives include a 10% reduction in the age-standardized death rate from 2007 (baseline) to 2020. The colorectal cancer target calls for a 15% reduction in death rates.
Trends in population risk, size, and age structure have been used to predict the future of cancer mortality in other countries (8). To determine whether HP2020 cancer mortality targets are likely to be met, we used mortality data and population estimates and projections to assess the contribution of changes in population risk, growth, and aging on cancer deaths from 1975 to 2020 for all cancer sites and the top 23 cancers by sex and race.
Methods
Mortality data were from the Centers for Disease Control and Prevention (CDC)’s National Vital Statistics System (9). Cause of death information was coded based on the International Classification of Diseases (ICD) versions in use at the time of death, and cause of death recodes were applied to accommodate consistency over time (2). For these analyses, we selected malignant neoplasms (C00–97). We used population estimates for 1975 through 2009 available from the SEER Program and obtained population projections for 2010 through 2020 from the US Census Bureau’s Population Projections Program (10).
To estimate the relative contribution to changes in the total number of new cancer deaths each year that can be attributed to changes in population risk (including changes in diagnosis and treatment practices) and demographic changes related to population size and age structure, we generated 3 sets of data by sex and race (white and black) for each year (1976–2009) based on a method first published in the 1999 Canadian Cancer Statistics report (11). The dotted line in Figure 1 represents the number of deaths from cancer that occurred in 1975. The lowest solid line represents the number of cancer deaths that would have occurred each year if the population size and age structure had remained the same as they were in 1975. This line is similar to the age-standardized death rate and reflects the impact of changes in population risk including changes in diagnosis and treatment practices. The middle line represents the number of deaths that would have occurred if the age structure of the population had remained the same as it was in 1975. This line reflects the impact of changes in risk and population growth. The top line represents the number of deaths that actually occurred and thus reflects the combined impact of changes in population risk, growth, and aging. The yearly difference between each set of death counts denotes the relative contribution to the overall change in the number of deaths since 1975 attributable to population risk, growth, and aging, respectively. A decline in risk during this time results in negative death counts as fewer deaths are attributed to risk compared with the baseline year.
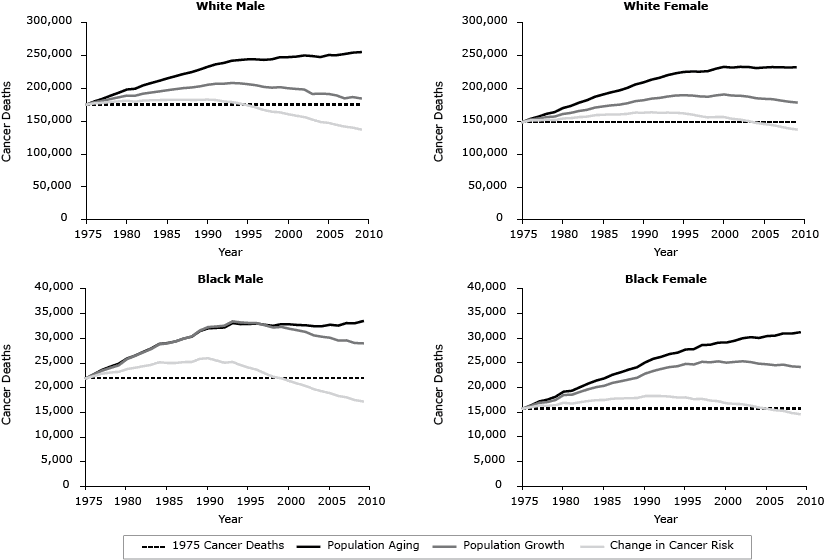
Figure 1. Trends in deaths from all cancers combined attributed to population risk (including diagnostic and treatment practices), growth, and aging (1975–2009), by sex and race (white, black). [A tabular version of this figure is also available.]
To project age-standardized death rates and counts for 2010–2020, we used Nordpred software (12), which uses an age-period-cohort regression model with data aggregated into six 5-year calendar periods (1980–2009) and fifteen 5-year age groups (15–19, …, 80–84, ≥85). We fit a separate model for each of the current top 23 cancer causes of death and all other cancer deaths combined by sex and race (all, white, and black): Rap = (Aa + D × p + Pp + Cc)5 where Rap is the death rate in age group a in calendar period p, Aa is the age component for age group a, D is the drift parameter (the common linear effect of both calendar period and birth cohort), Pp is the nonlinear period component of period p, and Cc is the nonlinear cohort component of cohort. To offset exponential increases or decreases in death rates, the models used the Power-5 link function. Assuming that trends are not likely to continue indefinitely, we reduced the drift component D by 25% and 50% in the second and third 5-year periods, respectively. Both these modifications have been shown empirically to improve predictions. Nordpred uses a goodness-of-fit test to determine the optimum number of calendar periods (4–6 candidate periods are sufficient) to include in the model (12). Significant curvature over time was determined by comparing a model with and without a second order term for calendar period (p), using a χ2 test for the difference in residual sum of squares between the models. If there was significant curvature, the slope from the last 10 years was projected; if not, the average linear trend (D) was used. Visual data inspection determined the starting age for each cancer site by sex and race such that each age group contained no fewer than 10 deaths. We calculated age-standardized death rates (per 100,000) using the US 2000 standard population weights (13). Predicted death counts were obtained by applying predicted age-specific death rates to the 2010 through 2020 population projections. We apportioned cancer death counts by changes in population risk and in demographics (population size and age structure combined) (14). We considered a change of 10% or more in age-adjusted rates or death counts as an increase or decrease; otherwise, we considered rates and death counts to be stable.
Results
From 1975 through 2009, the number of cancer deaths increased 45.5% among white males, 56.0% among white females, 52.8% among black males, and 98.2% among black females ( Table 1, Figure 1). For each sex and race group, the number of cancer deaths attributed to risk decreased, whereas the number of cancer deaths attributed to population growth and aging increased as follows: white males (−21.5% risk, 26.8% growth, 40.1% aging); white females (−7.6% risk, 27.6% growth, 36.0% aging); black males (−21.3% risk, 53.7% growth, 20.4% aging); and black females (−7.1% risk, 60.8% growth, 44.5% aging) (Table 1).
Figure 2 shows 1975 through 2009 (observed) and 2010 through 2020 (predicted) age-standardized cancer death rates for all sites combined and for the 7 site-specific cancers included in the cancer mortality objectives for Healthy People 2020. (Additional information can be found at http://www.cdc.gov/cancer/dcpc/research/articles/cancer_2020_figures.htm.) Table 2 shows predicted 2020 deaths by race (all races combined, white, black), sex, and cancer site, with the percentage change in deaths from 2007 (baseline) and 2020 apportioned because of changes in risk and demographics (population growth and aging combined). By 2020, we predict cancer deaths to increase 15.2% among men (−23.0% attributable to risk, 38.2% attributable to demographics) and begin to stabilize (8.1%) among women (−19.5% attributable to risk, 27.6% attributable to demographics). Results varied by cancer site, sex, and race. We predict that cancer risk will decrease for 13 of 19 cancers among men and 15 of 21 cancers in women, while the number of deaths will increase for 11 of 19 cancer sites among men and 9 of 21 cancer sites among women, in large part because of the demographic component. We predict that risk of death will increase for cancers of the corpus and uterus among black women, liver and intrahepatic bile duct among both men and women (both races), and for thyroid cancer in women (both races). We predict that the number of deaths for colorectal cancer and non-Hodgkin’s lymphoma will decrease among white women, for esophageal cancer among black men and women, Hodgkin’s lymphoma among white men and women, laryngeal cancer among black men, and cancers of the oral cavity and pharynx among black women.
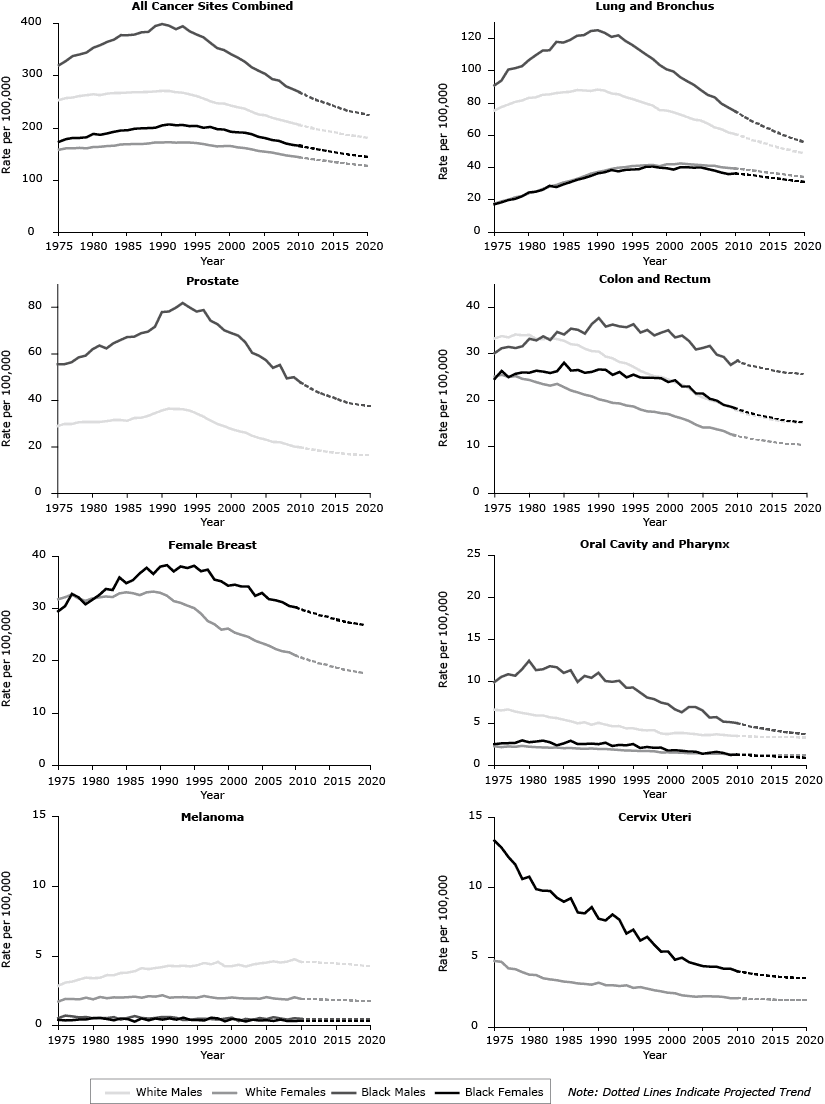
Figure 2. Trends in observed and predicted age-adjusted death rates for all sites combined and for the 7 site-specific cancers included in the Healthy People 2020 cancer mortality objectives by sex and race, 1975–2020. [A tabular version of this figure is also available.]
Table 3 shows observed (2007), predicted (2020), and HP2020 target age-standardized death rates per 100,000 population, percent change from 2007 to 2020, and the year in which we predict death rates to meet HP2020 targets by cancer site. We predict declining death rates from 2007 to 2020 for the following cancers: all sites combined (15.6%), lung and bronchus (21.3%), female breast (19.6%), cervix uteri (12.5%), colon and rectum (22.5%), oral cavity and pharynx (16.0%), prostate (26.4%), and melanoma of the skin (7.4%). The years in which we project the age-standardized death rate to meet the HP2020 target rates are 2010 (prostate), 2011 (cervix uteri, oral cavity and pharynx), 2012 (female breast), 2013 (lung and bronchus, colon and rectum), and 2015 (all sites combined). We do not predict that melanoma age-standardized death rates will meet the goal of a 10% reduction by 2020.
Discussion
Age-standardized death rates (population risk) began declining in the early 1990s and are predicted to continue to decline through 2020 for all cancer sites combined and for many of the leading cancers in both men and women. However, we predict that the total number of cancer deaths from 2007 to 2020 will increase more than 10% among men and black women and will begin to stabilize among white women, increasing only 4.4% during this period. Thus, while the overall risk of dying from cancer is declining, the impact of underlying demographic changes in the population will increase the burden of cancer on society and health care systems (15–17).
From 1975 to 2009, the observed number of cancer deaths increased among white Americans primarily because of an aging white population and among black Americans primarily because of a growing black population. Population aging has only recently begun to contribute to the increasing number of cancers deaths among black men. This shift can be explained by inequities in life expectancy. Compared with white Americans, life expectancy among black Americans in general and black men in particular remains lower because of higher death rates from heart disease, cancer, homicide, diabetes, and perinatal conditions (18). The demographic component underlying the increase in the number of cancer deaths is likely to continue into this century. Overall, the US population is expected to increase by 10% from 2010 to 2020; the largest increases are expected in minority populations and in the proportion of people older than 65 (13% to 16%) (3).
To help decrease the burden of cancer in the United States, HP2020 objectives called for a reduction in age-standardized death rates for all cancers combined, melanoma, and cancers of the female breast, cervix uteri, colon and rectum, lung and bronchus, oral cavity and pharynx, and prostate. In 2007, deaths from these cancers comprised the majority of cancer deaths (2). Many of these deaths could be prevented through reduced incidence of cancer, improved survival, or both. We predict that HP2020 target rates will be met first for prostate cancer in 2010 and lastly for all sites combined by 2015. We do not predict that melanoma cancer death rates will reach the HP2020 target rate by 2020. An examination of the most recent mortality data (9) shows that the HP2020 target for prostate cancer was met in 2010 and that the mortality rates for the other HP2020 targets declined as predicted with the exception of oral cavity and pharynx, which may be stabilizing.
An overarching goal of HP2020 is to improve the health of all population subgroups. These projections help inform the potential achievement of this goal. From 2007 to 2020, we predict a reduction of greater than 15% in death rates for colorectal cancer and a reduction of greater than 10% in death rates for cancers of the (female) breast, cervix, lung, oral cavity, and prostate, among white and black Americans. Despite this improvement, we expect that the total overall number of cancer deaths will increase in white men, black men, and black women and will begin to stabilize among white women. This trend reflects the impact of 2 leading cancers: we predict a modest 5.3% decline in the number of breast cancer deaths among white women, compared with a 15.3% increase among black women, and we predict that colorectal cancer deaths will decline by 10% among white women, stabilize among white men, and increase among black men and women.
The stabilization in deaths from lung cancer among men helps measure the success of primary prevention strategies aimed at reducing the incidence of cancer, particularly among highly fatal cancers. Incidence rates for lung cancer have been declining in parallel to a reduction in tobacco use (19). Among black women, the reduction in population risk is predicted to offset only partially the increase in lung cancer deaths attributable to demographic changes, and deaths are predicted to increase nearly 20% by 2020. Deaths from oral cancer are predicted to stabilize or decrease among women and black men during this period.
A reduction in the number of deaths from breast, cervical, and colorectal cancer among white women reflects the success of screening efforts and improved treatment. The reduction in age-standardized colorectal cancer death rates is consistent with a large reduction from screening and smaller reductions from risk factors and improved treatments (20). Access to quality health care, including earlier diagnosis and evidence-based treatments, has resulted in increased survival accompanied by reduced mortality for cancers of the colon and rectum and to a lesser extent, female breast and prostate (21).
Cancer projections can also alert researchers to the impact of changes in population risk before the full extent of the cancer burden manifests. This study identified several cancers in which increasing risk of death is exacerbating demographic trends, including cancers of corpus and uterus in black women, liver and intrahepatic bile duct cancer in both sexes and races, and thyroid cancer in women. Increasing cancer deaths, in part, reflect predicted increases in incidence rates of these cancers (22), possibly the result of a growing obesity epidemic (corpus and uterus cancer) (23), and an epidemic increase in hepatitis infections, particularly among those born from 1945 through 1965 (liver cancer) (24). The increase in deaths from thyroid cancer might be caused by unidentified risk factors operating at the population level and not the result of increased incidence associated with improved detection and access to care (25,26).
Since 1975, melanoma incidence rates have increased among all age groups, whereas death rates have remained unchanged overall and increased in older age groups (27). Melanoma deaths are predicted to increase through 2020 because incidence rates and risk are predicted to continue to increase (22), and death rates and risk are predicted to fall short of the HP2020 target of a 10% reduction from 2007 to 2020. To address the increasing melanoma burden, HHS issued The Surgeon General’s Call to Action to Prevent Skin Cancer, which includes strategies to reduce ultraviolet exposure from the sun and indoor tanning, thereby reducing melanoma incidence (28).
The estimates from our study are probably conservative, as the risk component in these models does not account for the potential for advances in primary prevention and treatment (29). Further reductions in cancer mortality, including melanoma, might yet be achievable if HP2020 objectives related to screening and access to health care are met (30).
This study used methods based on age-period cohort models that identify trends in younger birth cohorts and extrapolate these trends to future older cohorts (12,14). Although these methods have been validated in studies using long-term incidence data and are based on the best available information, they are subject to many known limitations. First, the population projections underlying the predicted death rates are themselves forecasts of the population size and age composition based on assumptions regarding future births, deaths, and migration. As such, these projections have the potential to impact cancer death and death rate projections. Second, the change in the number of cases between time periods has been allocated into changes because of risk, age structure, and population size. This allocation is arbitrary because the 3 components mutually affect each other. For example, if the population size increases, the effect of higher death rates (the risk component) will be larger than if the population size does not change. In the analysis of past trends, the first year (1975) was used as the reference year, following the approach in Canada (11). In the analysis of future trends, the final year (2020) was preferred from a preventive prospective (14). The consequence of using the final year as a reference rate is that the change in the number of deaths because of the combined effect of risk, age structure, and population size is attributed to risk, not demographics. If a future reduction in the risk of death can be achieved, the number of deaths from the combined effect of risk and demographics will be reduced.
Acknowledgments
There are no financial disclosures from any of the authors. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author Information
Corresponding Author: Hannah K. Weir, PhD, Epidemiology and Applied Research Branch, Division of Cancer Prevention and Control, Centers for Disease Control and Prevention, 4770 Buford Hwy, Mailstop F76, Atlanta, GA 30341. Telephone: 770-488-3006. Email: hbw4@cdc.gov.
Author Affiliations: Trevor D. Thompson, Steven Leadbetter, Mary C. White, Centers for Disease Control and Prevention, Atlanta, Georgia; Ashwini Soman, Northrop Grumman Corporation, Atlanta, Georgia; Bjorn Møller, Cancer Registry of Norway, Oslo, Norway.
References
- Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, et al. Annual report to the nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014;120(9):1290–314. CrossRef PubMed
- US Cancer Statistics Working Group. United States cancer statistics: 1999–2011 incidence and mortality web-based report. US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2013. http://apps.nccd.cdc.gov/uscs/. Accessed October 10, 2014.
- Vincent GK, Velkoff VA. The next four decades: the older population in the United States: 2010 to 2050. Current Population Report P25-1138. Washington (DC): US Census Bureau; 2010. http://www.census.gov/prod/2010pubs/p25-1138.pdf. Accessed October 6, 2014.
- Edwards BK, Howe HL, Ries LA, Thun MJ, Rosenberg HM, Yancik R, et al. Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on US cancer burden. Cancer 2002;94(10):2766–92. PubMed
- The National Cancer Act of 1971, Pub. L. 92-218, 85 Stat. 1828. (Dec 23, 1971). http://legislative.cancer.gov/history/phsa/1971. Accessed January 16, 2015.
- Major A, Stewart SL. Celebrating 10 years of the National Comprehensive Cancer Control Program, 1998 to 2008. Prev Chronic Dis 2009;6(4):A133. PubMed
- About Healthy People. Washington (DC): US Department of Health and Human Services; 2012. http://www.healthypeople.gov/2020/About-Healthy-People. Accessed October 22, 2014.
- Bray F, Møller B. Predicting the future burden of cancer. Nat Rev Cancer 2006;6(1):63–74. PubMed
- National Vital Statistics System (NVSS). Centers for Disease Control and Prevention. http://www.cdc.gov/nchs/nvss.htm. Accessed October 10, 2014.
- 2008 National population projections. US Census Bureau. https://www.census.gov/population/projections/data/national/2008.html. Accessed October 22, 2014.
- Canadian Cancer Society. Canadian Cancer Statistics 1999. Toronto (ON): 1999. http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-1999-EN.pdf. Accessed January 16, 2015.
- Møller B, Fekjaer H, Hakulinen T, Sigvaldason H, Storm HH, Talbäck M, et al. Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Stat Med 2003;22(17):2751–66. CrossRef PubMed
- Age adjustment using the 2000 projected US population. Bethesda (MD): National Center for Health Statistics, Centers for Disease Control and Prevention; 2001. http://www.cdc.gov/nchs/data/statnt/statnt20.pdf. Accessed May 15, 2014.
- Møller B, Fekjaer H, Hakulinen T, Tryggvadóttir L, Storm HH, Talbäck M, et al. Prediction of cancer incidence in the Nordic countries up to the year 2020. Eur J Cancer Prev 2002;11(Suppl 1):S1–96. PubMed
- Yang W, Williams JH, Hogan PF, Bruinooge SS, Rodriguez GI, Kosty MP, et al. Projected supply of and demand for oncologists and radiation oncologists through 2025: an aging, better-insured population will result in shortage. J Oncol Pract 2014;10(1):39–45. CrossRef PubMed
- Trogdon JG, Tangka FK, Ekwueme DU, Guy GP Jr, Nwaise I, Orenstein D. State-level projections of cancer-related medical care costs: 2010 to 2020. Am J Manag Care 2012;18(9):525–32. PubMed
- Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst 2011;103(2):117–28. CrossRef PubMed
- Kochanek KD, Arias E, Anderson RN. How did cause of death contribute to racial differences in life expectancy in the United States in 2010? NCHS Data Brief 2013;(125):1–8. PubMed
- Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 2008;100(23):1672–94. CrossRef PubMed
- Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116(3):544–73. CrossRef PubMed
- Cho H, Mariotto AB, Schwartz LM, Luo J, Woloshin S. When do changes in cancer survival mean progress? The insight from population incidence and mortality. J Natl Cancer Inst Monogr 2014;2014(49):187–97. CrossRef PubMed
- Weir HK, Thompson TD, Soman A, Møller B, Leadbetter S. The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer 2015;121(11):1827–37. CrossRef PubMed
- Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, et al. Annual Report to the Nation on the status of cancer, 1975-2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer 2012;118(9):2338–66. CrossRef PubMed
- Polednak AP. Surveillance and interpretation of trends in US age-specific incidence rates for primary liver cancer, in relation to the epidemic of hepatitis C infection. J Registry Manag 2013;40(3):115–21, quiz 144–5. PubMed
- Vollmer RT. Revisiting overdiagnosis and fatality in thyroid cancer. Am J Clin Pathol 2014;141(1):128–32. PubMed
- Morris LG, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid 2013;23(7):885–91. CrossRef PubMed
- Jemal A, Saraiya M, Patel P, Cherala SS, Barnholtz-Sloan J, Kim J, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol 2011;65(5, Suppl 1):S17–25.e1, 3. PubMed
- The Surgeon General’s call to action to prevent skin cancer. Washington (DC): US Department of Health and Human Services, Office of the Surgeon General; 2014. http://www.surgeongeneral.gov/library/calls/prevent-skin-cancer/. Accessed October 10, 2014.
- White MC, Peipins LA, Watson M, Trivers KF, Holman DM, Rodriguez JL. Cancer prevention for the next generation. J Adolesc Health 2013;52(5, Suppl):S1–7. CrossRef PubMed
- Brown ML, Klabunde CN, Cronin KA, White MC, Richardson LC, McNeel TS. Challenges in meeting Healthy People 2020 objectives for cancer-related preventive services, National Health Interview Survey, 2008 and 2010. Prev Chronic Dis 2014;11:E29. CrossRef PubMed
Tables
 Table 1. Observed Cancer Deaths (All Sites Combined) and Percentage Change From Population Risk, Growth, and Aging by Sex and Race, 1975–2009
Table 1. Observed Cancer Deaths (All Sites Combined) and Percentage Change From Population Risk, Growth, and Aging by Sex and Race, 1975–2009
| Death Year | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | Risk | Growth | Aging | Total | Risk | Growth | Aging | |
| White | ||||||||
| 1975 | 175,299 | NA | NA | NA | 148,711 | NA | NA | NA |
| 1980 | 198,189 | 5,994 | 7,635 | 9,261 | 169,970 | 5,486 | 6,781 | 8,993 |
| 1990 | 232,617 | 7,602 | 22,391 | 27,325 | 208,986 | 14,315 | 18,864 | 27,096 |
| 2000 | 247,396 | −14,631 | 39,464 | 47,265 | 232,608 | 7,558 | 34,396 | 41,943 |
| 2009 | 255,040 | −37,613 | 47,063 | 70,292 | 231,947 | −11,284 | 41,053 | 53,467 |
| % Change 1975–2009 | 45.5 | −21.5 | 26.8 | 40.1 | 56.0 | −7.6 | 27.6 | 36.0 |
| Black | ||||||||
| 1975 | 21,884 | NA | NA | NA | 15,747 | NA | NA | NA |
| 1980 | 25,855 | 1,841 | 2,024 | 106 | 19,173 | 1,193 | 1,562 | 671 |
| 1990 | 31,993 | 4,053 | 6,325 | −269 | 25,082 | 2,524 | 4,554 | 2,258 |
| 2000 | 32,815 | −592 | 10,615 | 908 | 29,127 | 1,072 | 8,228 | 4,080 |
| 2009 | 33,441 | −4,666 | 11,749 | 4,474 | 31,204 | −1,123 | 9,573 | 7,007 |
| % Change 1975–2009 | 52.8 | −21.3 | 53.7 | 20.4 | 98.2 | −7.1 | 60.8 | 44.5 |
Abbreviations: NA, not applicable.
 Table 2. Predicted (2020) Deaths and Percentage Change From 2007 to 2020 by Risk and Demographics (Population Growth and Aging Combined) for All Cancer Sites Combined and for the Top 23 Cancers by Race and Sex
Table 2. Predicted (2020) Deaths and Percentage Change From 2007 to 2020 by Risk and Demographics (Population Growth and Aging Combined) for All Cancer Sites Combined and for the Top 23 Cancers by Race and Sex
| Cancer Site | All Races | White | Black | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020, No. | % Change From 2007 to 2020 | 2020, No. | % Change From 2007 to 2020 | 2020, No. | % Change From 2007 to 2020 | |||||||||||||
| Overall | Attributable to | Overall | Attributable to | Overall | Attributable to | |||||||||||||
| Risk | Dem | Risk | Dem | Risk | Dem | |||||||||||||
| Males | ||||||||||||||||||
| All cancer sites combined | 337,280 | 15.2 | −23.0 | 38.2 | 285,800 | 13.4 | −21.4 | 34.8 | 38,248 | 15.7 | −32.7 | 48.3 | ||||||
| Brain and other CNS | 8,752 | 19.6 | −11.5 | 31.1 | 7,920 | 17.2 | −10.0 | 27.2 | 530 | 31.2 | −9.1 | 40.3 | ||||||
| Colon and rectum | 29,244 | 8.3 | −29.0 | 37.3 | 23,568 | 3.3 | −30.7 | 33.9 | 4,375 | 27.9 | −19.5 | 47.4 | ||||||
| Esophagus | 14,313 | 33.1 | −4.9 | 38.0 | 13,010 | 37.1 | 2.9 | 34.1 | 899 | −14.8 | −65.3 | 50.6 | ||||||
| Hodgkin lymphoma | 680 | −3.7 | −29.5 | 25.8 | 553 | −11.0 | −33.9 | 22.9 | 72 | −2.5 | −32.1 | 29.6 | ||||||
| Kidney and renal pelvis | 9,788 | 22.9 | −14.4 | 37.3 | 7,932 | 13.4 | −20.4 | 33.7 | 1,032 | 38.7 | −7.3 | 46.0 | ||||||
| Larynx | 3,047 | 5.4 | −31.9 | 37.3 | 2,512 | 10.0 | −23.6 | 33.7 | 468 | −14.5 | −63.6 | 49.1 | ||||||
| Leukemia | 15,006 | 20.7 | −15.5 | 36.2 | 13,278 | 18.7 | −14.4 | 33.1 | 1,224 | 27.9 | −14.7 | 42.6 | ||||||
| Liver and IBD | 20,757 | 83.0 | 49.8 | 33.2 | 16,216 | 82.5 | 52.8 | 29.7 | 2,983 | 87.4 | 42.8 | 44.6 | ||||||
| Lung and bronchus | 91,592 | 3.7 | −37.1 | 40.8 | 78,588 | 2.8 | −34.4 | 37.2 | 9,817 | −0.2 | −51.6 | 51.4 | ||||||
| Melanoma | 6,728 | 22.2 | −11.4 | 33.6 | 6,546 | 21.0 | −8.6 | 29.6 | 81 | 36.7 | −4.5 | 41.2 | ||||||
| Myeloma | 6,831 | 17.6 | −22.0 | 39.5 | 5,403 | 12.8 | −23.6 | 36.4 | 1,157 | 32.5 | −16.1 | 48.7 | ||||||
| Non-Hodgkin lymphoma | 10,662 | −3.1 | −39.9 | 36.8 | 9,369 | −5.8 | −39.5 | 33.7 | 893 | 19.2 | −23.9 | 43.1 | ||||||
| Oral cavity and pharynx | 6,216 | 12.8 | −22.4 | 35.2 | 5,389 | 18.3 | −13.4 | 31.7 | 698 | −9.2 | −55.8 | 46.6 | ||||||
| Pancreas | 24,339 | 42.1 | 3.6 | 38.4 | 20,674 | 39.9 | 5.1 | 34.8 | 2,541 | 34.2 | −14.3 | 48.6 | ||||||
| Prostate | 30,914 | 6.3 | −33.9 | 40.2 | 24,778 | 4.7 | −32.4 | 37.1 | 5,285 | 7.7 | −41.6 | 49.3 | ||||||
| Stomach | 6,857 | 1.5 | −35.3 | 36.8 | 4,951 | −2.9 | −36.4 | 33.5 | 1,264 | 6.0 | −40.5 | 46.5 | ||||||
| Testis | 356 | 9.1 | −3.4 | 12.4 | 315 | 6.9 | −1.5 | 8.3 | — | — | — | — | ||||||
| Thyroid | 967 | 39.3 | 1.0 | 38.3 | 841 | 39.2 | 4.8 | 34.4 | — | — | — | — | ||||||
| Urinary bladder | 13,113 | 36.0 | −3.3 | 39.3 | 11,946 | 33.5 | −2.8 | 36.2 | 887 | 56.9 | 8.9 | 48.0 | ||||||
| Females | ||||||||||||||||||
| All cancer sites combined | 291,923 | 8.1 | −19.5 | 27.6 | 242,196 | 4.4 | −18.4 | 22.8 | 36,392 | 17.5 | −24.9 | 42.3 | ||||||
| Brain and other CNS | 6,771 | 14.4 | −12.0 | 26.4 | 6,050 | 11.7 | −10.1 | 21.7 | 438 | 13.2 | −24.1 | 37.3 | ||||||
| Cervix uteri | 4,085 | 1.6 | −17.7 | 19.3 | 3,032 | −0.2 | −14.3 | 14.1 | 856 | 6.3 | −26.5 | 32.8 | ||||||
| Colon and rectum | 24,887 | −5.1 | −30.2 | 25.1 | 19,834 | −10.0 | −30.1 | 20.2 | 3,802 | 10.1 | −32.9 | 42.9 | ||||||
| Corpus and uterus, NOS | 10,407 | 39.6 | 8.7 | 30.9 | 7,847 | 31.6 | 6.7 | 25.0 | 2,092 | 61.6 | 10.6 | 50.9 | ||||||
| Esophagus | 3,004 | 5.7 | −23.7 | 29.4 | 2,672 | 12.1 | −12.5 | 24.6 | 339 | −14.9 | −58.7 | 43.9 | ||||||
| Female breast | 40,434 | −0.4 | −25.5 | 25.0 | 32,065 | −5.3 | −25.8 | 20.6 | 6,683 | 15.3 | −21.6 | 36.9 | ||||||
| Hodgkin lymphoma | 490 | −13.2 | −35.3 | 22.0 | 424 | −15.3 | −33.1 | 17.8 | 55 | −2.5 | −33.6 | 31.1 | ||||||
| Kidney and renal pelvis | 5,136 | 8.4 | −19.5 | 27.9 | 4,382 | 5.7 | −17.6 | 23.3 | 516 | 8.0 | −33.6 | 41.6 | ||||||
| Larynx | 863 | 15.9 | −16.0 | 31.9 | 747 | 20.1 | −6.1 | 26.2 | 118 | 5.8 | −44.1 | 49.9 | ||||||
| Leukemia | 10,180 | 7.2 | −17.6 | 24.8 | 8,825 | 5.3 | −14.9 | 20.2 | 923 | 3.7 | −36.2 | 39.9 | ||||||
| Liver and IBD | 8,576 | 47.8 | 20.7 | 27.1 | 6,853 | 44.9 | 22.5 | 22.4 | 1,046 | 55.6 | 13.8 | 41.8 | ||||||
| Lung and bronchus | 76,679 | 9.0 | −22.7 | 31.7 | 65,793 | 5.8 | −21.1 | 26.8 | 7,926 | 19.2 | −26.6 | 45.7 | ||||||
| Melanoma | 3,361 | 13.7 | −9.5 | 23.2 | 3,195 | 12.1 | −6.1 | 18.2 | 82 | 6.3 | −34.3 | 40.5 | ||||||
| Myeloma | 4,811 | −5.0 | −34.0 | 29.1 | 3,611 | −8.7 | −32.6 | 24.0 | 947 | −5.0 | −51.4 | 46.3 | ||||||
| Non-Hodgkin lymphoma | 7,831 | −17.8 | −43.2 | 25.5 | 6,813 | −21.1 | −42.1 | 20.9 | 641 | 0.0 | −39.9 | 39.8 | ||||||
| Oral cavity and pharynx | 2,615 | 2.3 | −23.5 | 25.8 | 2,230 | 3.0 | −18.5 | 21.5 | 232 | −20.9 | −61.0 | 40.1 | ||||||
| Ovary | 16,432 | 12.4 | −16.3 | 28.7 | 14,122 | 7.9 | −15.9 | 23.7 | 1,398 | 20.5 | −24.0 | 44.5 | ||||||
| Pancreas | 23,117 | 36.1 | 7.9 | 28.2 | 18,999 | 32.7 | 9.4 | 23.3 | 2,981 | 37.0 | −7.9 | 44.9 | ||||||
| Stomach | 4,460 | −3.7 | −27.8 | 24.1 | 3,159 | −8.5 | −27.4 | 18.9 | 822 | 2.1 | −39.5 | 41.5 | ||||||
| Thyroid | 1,297 | 49.4 | 21.2 | 28.2 | 1,006 | 41.5 | 18.8 | 22.7 | 172 | 82.5 | 34.9 | 47.7 | ||||||
| Urinary bladder | 4,961 | 18.1 | −6.1 | 24.2 | 4,289 | 16.9 | −3.0 | 19.9 | 544 | 16.1 | −25.0 | 41.1 | ||||||
Abbreviations: —, Data not shown because numbers were too small for analysis; Dem, demographics.
 Table 3. Observed (2007) and Predicted (2020) Age-Standardized Death Rates (ASDR) per 100,000 Population and Overall Percentage Change for All Cancer Sites Combined and for the 7 Site-Specific Cancers Included in the Healthy People 2020 Cancer Mortality Objectives
Table 3. Observed (2007) and Predicted (2020) Age-Standardized Death Rates (ASDR) per 100,000 Population and Overall Percentage Change for All Cancer Sites Combined and for the 7 Site-Specific Cancers Included in the Healthy People 2020 Cancer Mortality Objectives
| HP2020 Objectives/Cancer Site | 2007 Observed ASDR | 2020 Predicted ASDR | Percentage Change (2007–2020) ASDR | HP2020 Target ASDR | Year Predicted to Meet HP2020 Target |
|---|---|---|---|---|---|
| C-1, All cancer sites combined | 179.3 | 151.4 | −15.6 | 161.4 | 2015 |
| C-2, lung and bronchus | 50.7 | 39.8 | −21.3 | 45.5 | 2013 |
| C-3, female breast | 23.0 | 18.5 | −19.6 | 20.7 | 2012 |
| C-4, cervix uteri | 2.4 | 2.1 | −12.5 | 2.2 | 2011 |
| C-5, colon and rectum | 16.9 | 13.1 | −22.5 | 14.5 | 2013 |
| C-6, oral cavity and pharynx | 2.5 | 2.1 | −16.0 | 2.3 | 2011 |
| C-7, prostate | 24.2 | 17.8 | −26.4 | 21.8 | 2010 |
| C-8, melanoma | 2.7 | 2.5 | −7.4 | 2.4 | — |
Abbreviations: —, will not achieve goal of a 10% reduction by 2020; HP2020, Healthy People 2020.
- Page last reviewed: July 2, 2015
- Page last updated: July 2, 2015
- Content source:
- Maintained By:


 ShareCompartir
ShareCompartir