Protecting People and Preventing Complications of Blood Disorders
 CDC’s National Center on Birth Defects and Developmental Disabilities (NCBDDD) is reducing the consequences of blood disorders by contributing to a better understanding of blood disorders and their complications.
CDC’s National Center on Birth Defects and Developmental Disabilities (NCBDDD) is reducing the consequences of blood disorders by contributing to a better understanding of blood disorders and their complications.
Blood disorders affect millions of people in the United States. Patients with blood disorders live with the complications associated with their conditions, many of which are painful and potentially life threatening.
NCBDDD’s Division of Blood Disorders (DBD) is committed to helping people with blood disorders. We contribute to a better understanding of blood disorders while working to develop, implement, and evaluate programs that help consumers and healthcare providers get critical information on preventing complications. We encourage action to improve the quality of life for people living with these conditions. With proper preventive actions and early medical intervention, many of these disorders and their complications could be eliminated.
Budget and Funding
Accomplishments
- Launched Phase 2 of the Stop the Clot, Spread the Word Campaign, an important public health campaign to share information about blood clot risks and the signs and symptoms of blood clots. Phase 2 of the campaign targets patients recently hospitalized, those undergoing surgery, and victims of physical trauma. Campaign assets include web content, a video, an infographic, a radio news release, a mat release (an online feature article), a digital multimedia press release, and a Department of Health and Human Services (DHHS) blog.
- Recognized eight public and private healthcare practices and systems across the country as Healthcare-Associated Venous Thromboembolism (HA-VTE) Prevention Challenge Champions for their success in helping doctors prevent blood clots in their patients that occur as a result of hospitalization, surgery, or other healthcare treatment. CDC is sharing these best practices with others to help strengthen venous thromboembolism (VTE) prevention efforts.
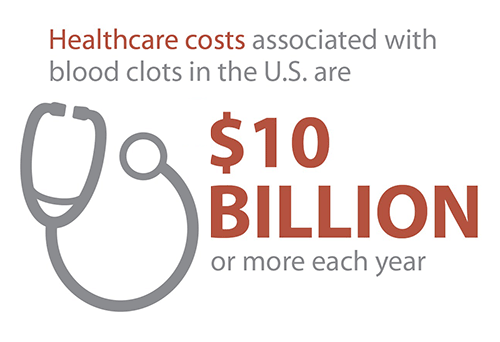
- Performed inhibitor testing as part of the Community Counts Registry for Bleeding Disorders Surveillance, and provided the first ever estimates of the burden of inhibitors in the United States hemophilia population. An inhibitor is a potentially serious health problem affecting people with bleeding disorders that stops or “inhibits” their treatment product from working, which makes it harder to stop or prevent a bleeding episode. Inhibitor testing strengthens prevention efforts by diagnosing inhibitors early, when they are more likely to be successfully eliminated using special treatment protocols.
- Worked with funded partners to implement the Community Counts project in hemophilia treatment centers (HTCs) across the United States, raising participation among all 136 federally-funded HTCs (as of July 31, 2016) to 100% for the HTC Population Profile, 92% for the Registry for Bleeding Disorders Surveillance, and 91% for Mortality Reporting.
- Supported and coordinated the development and promotion of a supplement issue of the American Journal of Preventive Medicine on sickle cell disease (SCD), entitled “Developing a Unified Approach for Sickle Cell Disease.” Articles in the supplement describe the state of sickle cell disease-related care in the United States. The articles highlight the public health impact of SCD on patients, healthcare providers and healthcare systems, and all the critical scientific work that is being done to reduce the burden of SCD on people living with the condition, as well as their families.
Looking to the Future
NCBDDD’s Division of Blood Disorders (DBD) will continue to learn more about the factors that place people with hemophilia at risk for developing inhibitors. DBD will study and promote the use of periodic testing to find out if a person has an inhibitor, monitor complications from blood and treatment products, and research how well various treatment strategies prevent disease-related complications. We will focus on preventing blood clots that occur as a result of hospitalization, surgery, or other healthcare treatment. Also, we will work to improve laboratory techniques for inhibitor testing and for identifying factors that increase the risk for VTE. Lastly, DBD will focus on increasing healthcare provider and public knowledge and awareness regarding signs and symptoms of blood clots. In addition, we will continue to emphasize the importance of early detection and diagnosis, and referrals to medical specialists and comprehensive care (an approach that uses the services of numerous professionals working together to provide for a patient’s needs, not just the medical and physical ones). DBD will continue research to understand complications of blood transfusions used to treat people with thalassemia and SCD.
Notable 2016 Scientific Publications
Boylan B, et al. Survey of the anti-factor IX immunoglobulin profiles in patients with hemophilia B using a fluorescence-based immunoassay. J Thromb Haemost. 2016 Oct;14(10):1931-40.
Learn about NCBDDD’s Blood Disorders Topics
Dargaud Y, et al. Achievements, challenges and unmet needs for haemophilia patients with inhibitors: Report from a symposium in Paris, France on 20 November 2014. Haemophilia. 2016 Jan;22(Suppl 1):1-24.
Dupervil B, et al. Correction: Emergency department visits and inpatient admissions associated with priapism among males with sickle cell disease in the United States, 2006–2010. PloS One. 2016 Aug 24;11(8):e0162056.
Mazepa M, et al. US Hemophilia Treatment Center Network. Men with severe hemophilia in the United States: Birth cohort analysis of a large national database. Blood. 2016 Jun 16;127(24):3073-81.
Pai M, et al. NHF-McMaster Guideline on Care Models for Haemophilia Management. Haemophilia. 2016 Jul;22(Suppl 3):6-16.
Paulukonis S, et al. Defining sickle cell disease mortality using a population-based surveillance system, 2004 through 2008. Public Health Rep. 2016 Mar-Apr;131(2):367-75.
Yeung CH, et al. Care models in the management of haemophilia: A systematic review. Haemophilia. 2016 Jul;22(Suppl 3):31-40.
Spotlight On: American Thrombosis and Hemostasis Network (ATHN)
This Spotlight was contributed by Becky Dudley, Director of National Surveillance at ATHN.
The American Thrombosis and Hemostasis Network (ATHN) is a nonprofit organization dedicated to advancing and improving care for people with bleeding and clotting disorders. Founded in 2006, ATHN’s mission provides stewardship of a secure national database (adherent to all privacy guidelines), which can be used to support clinical care and outcome analysis, research, advocacy, and public health surveillance. ATHN’s ultimate goal is improving lives using standardized technology to secure data, advance knowledge, and transform care.

ATHN partners with the bleeding disorders community, government, and industry, and its ATHN Affiliates—the more than 135 hemophilia treatment centers (HTCs) across the United States that provide multi-disciplinary, comprehensive care to more than 32,500 patients annually. Through CDC’s Community Counts Registry for Bleeding Disorders Surveillance, ATHN collaborates with CDC and HTCs to identify the number of people with bleeding and clotting disorders in the United States and monitor trends that will help prevent complications of these disorders. Together with the HTCs, ATHN is building the ATHNdataset—a safe, secure national dataset that has more than 30,000 patients who have opted to share their clinical and demographic data to support vital research.
ATHN provides funding for national data collection and research efforts on bleeding and clotting disorders. By leveraging its standardized integrated systems, data, and processes, ATHN helps decrease the time and cost for research and public health surveillance related to complications of bleeding disorders, such as inhibitors and hepatitis C, joint disease, venous thromboembolism (VTE), and cardiovascular and renal disease.
ATHN’s many current projects include My Life, Our Future: Genotyping for Progress in Hemophilia; ATHN 2 – Factor Switching Study; ATHN 4 – Transition of Care for VTE Patients; ATHN 5 – Hepatitis C Treatment Outcomes; and quality initiatives led by the National Hemophilia Program Coordinating Center, which ATHN established in 2012 to identify gaps in services, standardize and increase access to care, and improve quality of care for those with bleeding disorders.
A multi-stakeholder Board of Directors representing ATHN Affiliates, regional leaders, and consumers govern ATHN. CDC, the Health Resources and Services Administration Maternal and Child Health Bureau, and the National Heart, Lung and Blood Institute provide input through liaisons to the Board and key committees. As committed stewards of the most current and extensive hemostasis and thrombosis data and analytic resources, and with results from future research and surveillance, ATHN promises to advance knowledge and transform care for patients with bleeding and clotting disorders.
- Page last reviewed: January 24, 2017
- Page last updated: January 24, 2017
- Content source:


 ShareCompartir
ShareCompartir

