 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Imported Wild Poliovirus Causing Poliomyelitis --- Bulgaria, 2001In March 2001, a 13-month-old unvaccinated Roma (i.e., gypsy) girl from Bourgas, Bulgaria, had onset of bilateral leg weakness. The National Enterovirus Laboratory in the capital city of Sofia subsequently isolated a wild type 1 poliovirus in the patient's stool. In April, a second case, with wild type 1 poliovirus isolate was found in Iambol located approximately 50 miles west of Bourgas in an unvaccinated 26-month-old Roma girl who had onset of paralysis of both legs. Subsequent analyses indicated that these viruses were related closely to a strain isolated from Uttar Pradesh, India, in July 2000. A third confirmed case with clinical and serologic evidence of poliomyelitis was diagnosed in a 3-month-old Roma boy in Bourgas who had onset of paralysis on May 7. Following the identification of the poliovirus, the Bulgarian Ministry of Health implemented contact investigations, screening of children at high risk, retrospective record review, intensified acute flaccid paralysis (AFP) surveillance, and mass vaccinations. This report summarizes the outbreak investigation and supplemental vaccination activities in response to these polio cases. High routine vaccination coverage and certification standard AFP surveillance are necessary to detect rapidly and prevent the spread of poliovirus importations in areas and countries where polio is not endemic. During 1998--2000, AFP surveillance in Bulgaria had detected 0.9 nonpolio cases per 100,000 persons aged <15 years per year (adequate surveillance is indicated by a nonpolio AFP case detection of >1 per 100,000 persons aged <15 years). In addition, 79% of AFP cases were investigated with adequate stool specimens* (adequate performance is indicated by an adequate specimen collection rate of at least 80%). During January--March 2001, two AFP cases were detected in Bulgaria. Following identification of case 1, the number of AFP cases identified increased rapidly. As of November 1, a total of 33 cases had been identified, including 30 nonpolio cases, corresponding to a nonpolio AFP detection rate of 2.6 per 100,000 persons aged <15 years. The proportion of cases with adequate specimens was 94%. During April--May 2001, serosurveys were conducted among high-risk children (i.e., children from minority communities or residing close to areas with large minority populations) aged 0--83 months. Among 26 Roma children hospitalized in Bourgas, 12 (46%) lacked detectable antibodies (Table 1). High-risk children from Sofia were more likely to lack antibodies to all three types of polioviruses (nine of 12 children) than children residing in Dobrich, Pazardjik, and Plovdiv (six of 33 children). Stool specimens also were obtained from children at high risk for exposure. Wild type 1 poliovirus was found in an 11-month-old girl in Karnobat whose sister had shared the hospital ward with case 1, and in a 15-month-old girl in Sofia. These children had no symptoms compatible with polio. To control the outbreak, a mass vaccination campaign of high-risk children was initiated on April 19 in the area of residence of case 1 and was expanded to the entire Bourgas district and the three neighboring districts of Iambol, Sliven, and Stara Zagora on April 27. During May 28--June 1 and June 25--29, 2001, a national campaign composed of two rounds with a goal of vaccinating all 468,720 children aged 0--6 years was conducted. Administrative coverage estimates suggested that 94% of all children in the country were vaccinated during the first round and 95% during the second. Because the initial contact investigations revealed that up to half of the children from high-risk groups were not vaccinated fully by the routine program, one additional round of mass vaccination was conducted during October for high-risk children aged 0--4 years; another round is scheduled for November. Reported by: A Kuntchev, Ministry of Health; M Kojuharova, Dept of Epidemiology; S Gjurova, N Korsum, National Enterovirus Laboratory, National Center for Infectious and Parasitic Diseases, Sofia, Bulgaria. L Fiore, Regional Poliomyelitis Reference Laboratory, Rome, Italy. Regional Office for Europe, World Health Organization, Copenhagen, Denmark. Vaccines and Other Biologicals Dept, World Health Organization, Geneva, Switzerland. Respiratory and Enterovirus Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Global Immunization Div, National Immunization Program, CDC. Editorial Note:This report describes the transmission for several months of a wild poliovirus imported into a country that had been free of polio for approximately 10 years. This outbreak of polio occurred because poliovirus was introduced into population subgroups with low immunity. The last indigenous wild poliovirus in the 51-country European Region (EUR) of the World Health Organization (WHO) occurred in November 1998 in Turkey (1). The last outbreak of polio in Bulgaria occurred in 1991 and involved 46 confirmed cases from the Roma community (2). Suboptimal immunity in the Roma population contributed to the 1991 and 2001 outbreaks. Population subgroups with lower vaccination coverage can sustain the circulation of wild polioviruses for several years within a country (3--5). High-risk communities are present in all European countries. As polio is eliminated, areas or population groups with lower immunity remain vulnerable to importation of wild poliovirus and subsequent transmission (6,7). When wild poliovirus type 1 was confirmed in this outbreak, WHO immediately informed authorities in all EUR member states and asked them to enhance AFP surveillance and rapidly enhance vaccination coverage in hard-to-reach minority population subgroups. WHO conducted training and consultation to improve surveillance and vaccination in several countries neighboring Bulgaria. Bulgarian authorities promptly implemented National Immunization Days§ within 64 days of paralysis onset in case 1. High coverage reported for the campaign countrywide, improved performance of AFP surveillance, and the absence of wild polioviruses in subsequent stool surveys of high-risk children suggest that circulation of the wild virus has been interrupted. The investigations and interventions by the Bulgarian Ministry of Health exemplify an effective response to possible importation of poliovirus that is particularly useful as EUR prepares to certify eradication of polio. Until polio is eradicated, the risk for importation will persist in countries and areas free of polio. References
* Two stool specimens collected at least 24 hours apart within 14 days of onset of paralysis and shipped adequately to the laboratory. Vaccination coverage determined by the administrative method (in which the doses administered is the numerator and the estimated number of children to be vaccinated is the denominator) is often higher than coverage determined through surveys because of overestimates in the number of doses of vaccine administered and underestimates of the size of the population that should receive vaccination. § Mass campaigns over a period of days to weeks in which two doses of oral poliovirus vaccine are administered to all children usually aged <5 years regardless of previous vaccination history with an interval of 4--6 weeks between doses. Table 1 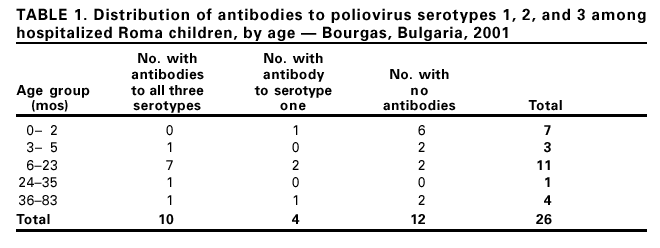 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 11/23/2001 |
|||||||||
This page last reviewed 11/23/2001
|