 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Progress Toward Poliomyelitis Eradication --- European Region, 1998--June 2000In 1988, the World Health Assembly resolved to eradicate poliomyelitis globally by 2000 (1). Substantial progress has been made since 1995, when the World Health Organization (WHO) European Region (EUR), comprising 51 member states (including Israel and the Central Asian Republics), accelerated efforts toward polio eradication (2--4). This report summarizes progress toward polio eradication during 1998--June 2000, and suggests that indigenous transmission of wild poliovirus has been interrupted in EUR. Routine vaccination coverage. In 1999, 38 EUR countries routinely used oral poliovirus vaccine (OPV) for infant vaccination, seven used inactivated poliovirus vaccine (IPV), and six used sequential IPV--OPV schedules. In 1998, the regional average for coverage with a primary series of polio vaccination by age 1 year was 94% (range: 77%--100%, with 26 countries reporting), compared with 83% in 1993 (range: 45%--100%, with 46 countries reporting); coverage levels in many of the Newly Independent States of the Former Soviet Union improved to pre-independence levels after reaching their lowest points during the economic transitions of the early 1990s. Supplemental vaccination activities. From 1995 to 1997, National Immunization Days (NIDs)* were conducted in 18 contiguous countries of the WHO Eastern Mediterranean (Afghanistan, Islamic Republic of Iran, Iraq, Jordan, Lebanon, Pakistan, Palestinian Authority, and Syrian Arab Republic) and European regions (Armenia, Azerbaijan, Georgia, Kazakhstan, Kyrgyzstan, Russian Federation, Tajikistan, Turkey, Turkmenistan, and Uzbekistan) as part of "Operation MECACAR" (Eastern Mediterranean, Caucasus, and Central Asian Republics). Reported coverage with two doses of OPV was >95% in each year (2). Beginning in the fall of 1997 with "mopping-up" vaccination, coordinated activities in countries of the two regions continued as "Operation MECACAR Plus". In 1998, all MECACAR countries participated in NIDs. Since 1999, activities have been more limited; sub-NIDs or supplemental vaccination programs were not conducted in some MECACAR countries of EUR. NIDs were conducted during April--May 2000 in Tajikistan, Turkey, Turkmenistan, and Uzbekistan, and sub-NIDs in Armenia, Azerbaijan, and Russian Federation, with reported coverage >93% for each round, and sub-NIDs in Bosnia and Herzegovina, with coverage >90%. Since fall 1998, the quality of supplemental vaccination in high-risk eastern and southeastern provinces of Turkey has improved dramatically because of improved provincial planning, house-to-house vaccination, supervision, and social mobilization. Surveillance. By 1997, all 17 countries where polio was recently endemic (i.e., polio cases reported since 1992) had established AFP surveillance (Table 1). In addition, 22 countries where polio is not endemic also routinely reported AFP surveillance data. From January 1999 through June 2000, all but three of the 17 countries where polio was recently endemic (Albania, Azerbaijan, and Bosnia and Herzegovina) have achieved the minimum AFP reporting rate indicative of sensitive surveillance (>1 nonpolio AFP case per 100,000 children aged <15 years annually). Although the quality of AFP surveillance has varied in some countries where polio was recently endemic, many have consistently reported rates >1 since 1998. The overall collection rate for two adequate stool samples§ from AFP case-patients in countries where polio was recently endemic increased from 78% in 1998 to 88% by June 2000 (Table 1). During 1999--2000, most countries consistently achieved the WHO-recommended target of two adequate stool specimens collected from at least 80% of persons with AFP. Training and assessment programs have been conducted since 1997, with resources focused on improved monitoring, supervision, and active surveillance. Since 1999, emphasis has been placed on monitoring AFP surveillance performance of lower administrative levels within countries where polio was recently endemic, enabling more appropriate tailoring of corrective interventions. Since 1999, all 39 countries conducting AFP surveillance are reporting case-based AFP surveillance data weekly to the WHO regional office. By June 2000, completeness of reports received for weekly reporting was 86% and timeliness of reporting was 82%. EUR laboratory network. The EUR polio laboratory network consists of 39 laboratories: 32 national, one subregional, and six regional reference laboratories (four serve also as national laboratories). Annual WHO accreditation of national laboratories is ongoing (4); 36 (92%) network laboratories have received full accreditation. All AFP cases reported in 2000 have been processed in fully accredited laboratories. The timeliness of specimen transport to national laboratories has been inadequate in nine countries where <50% of specimens reached a national laboratory within 3 days of collection. Incidence of polio. From 1991 through 1996, the number of confirmed polio cases¶ reported annually in EUR ranged from 177 to 297; in 1997, seven cases from two countries (Tajikistan and Turkey) were reported. During February--November 1998, wild poliovirus type 1 was isolated in 24 cases and wild poliovirus type 3 in two cases in eight eastern and southeastern provinces of Turkey. The last reported case occurred in Agri province with paralysis onset on November 26, 1998. Certification process. The European Regional Commission for the Certification of Poliomyelitis Eradication has begun reviewing documentation on the vaccination and surveillance activities of EUR countries. Forty-nine member states have formed national certification committees to review country vaccination, laboratory, and epidemiologic surveillance data and submit documentation to the regional commission. Documentation from 32 countries of Europe where no polio cases have been reported for >8 years was initially reviewed during 1998--1999; countries where polio was recently endemic will be reviewed during 2000--2001. In addition, a process was initiated in 1999 for registering, containing, and/or destroying any wild poliovirus isolates or potentially infectious material (5). Reported by: Communicable Diseases Unit, World Health Organization Regional Office for Europe, Copenhagen, Denmark. Dept of Vaccines and Biologicals, World Health Organization, Geneva, Switzerland. Respiratory and Enteric Viruses Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Vaccine Preventable Disease Eradication Div, National Immunization Program, CDC. Editorial Note:Indigenous poliovirus transmission probably was interrupted in EUR countries in 1998; this status is attributed to improvements in routine vaccination coverage and the successful implementation of coordinated supplemental vaccination through Operation MECACAR and MECACAR Plus. In addition, AFP surveillance in nearly all EUR countries where polio was recently endemic has improved substantially. Along with continued observation, the quality of surveillance and timely transport of specimens in some areas of the region need further improvement to document that indigenous transmission has been interrupted and that any transmission secondary to imported poliovirus is detected promptly. Strengthening of surveillance and specimen transport is particularly important in some areas of Turkey. Eastern and southeastern areas of Turkey adjacent to Syria, Iran, and Iraq remain at high risk for wild poliovirus transmission; wild polioviruses have been isolated from AFP cases in Iraq during 1999 and in early 2000 (4,6). Although cross-border travel is generally prohibited and tightly monitored, Tajikistan, Turkmenistan, and Uzbekistan remain at risk for polio because of ongoing poliovirus transmission in neighboring Afghanistan (7). Interregional and intercountry efforts are ongoing to coordinate surveillance and supplementary vaccination activities in key high-risk border areas. Supplemental vaccination activities will be needed at least through 2002 in Tajikistan, Turkey, Turkmenistan, and Uzbekistan under Operation MECACAR Plus. This activity will be coordinated with bordering Eastern Mediterranean Region (EMR) countries and include mopping-up campaigns in October and November 2000 to ensure interruption of any remaining chains of poliovirus transmission and to impede circulation in the case of reintroduction of virus. EUR priorities include 1) maintaining and strengthening AFP surveillance systems, particularly in the Caucasus, Turkey, and the Central Asian Republics; 2) conducting high-quality NIDs or sub-NIDs through Operation MECACAR Plus in selected countries with persistent high risk for wild poliovirus circulation, in coordination with bordering EMR countries; 3) implementing coordinated house-to-house supplemental vaccination activities among key border area populations; 4) maintaining and strengthening the political commitment of governments for polio eradication and certification; 5) consolidating the support of donor governments and partner agencies to ensure sufficient financial and human resources**; and 6) implementing laboratory containment of wild poliovirus and potentially infectious materials. These activities will ensure that the interruption of poliovirus transmission is maintained and that the region can be certified as polio-free by 2003. References
* Mass campaigns over a short period (days to weeks) in which two doses of OPV are administered to all children in the target age group, regardless of previous vaccination history, with an interval of 4--6 weeks between doses. Focal mass campaign in high-risk areas over a short period (days to weeks) in which two doses of OPV are administered during house-to-house visits to all children in the target age group, regardless of previous vaccination history, with an interval of 4--6 weeks between doses. § Two stool specimens collected within 14 days of onset of paralysis at an interval of at least 24 hours. WHO recommends that >80% of patients with AFP have two adequate specimens collected. ¶ A confirmed case of polio is defined under the virologic scheme of classification as AFP with laboratory-confirmed wild poliovirus infection; in countries where virologic surveillance is inadequate, clinical cases have either residual paralysis at 60 days, death, or no follow-up investigation at 60 days. Since 1997, all countries in EUR but Tajikistan have used the virologic scheme of classification of AFP cases, for which some AFP cases with residual paralysis at 60 days, death, or no follow-up investigation may be considered as polio-compatible cases. Since 1999, the virologic classification scheme has been applied throughout EUR. ** Polio eradication efforts in EUR have been supported by the governments of countries where polio was recently endemic, WHO, United Nations Children's Fund (UNICEF), Rotary International, U.S. Agency for International Development, the Japanese International Cooperation Agency, the United Nations Foundation, CDC, and other countries. Table 1 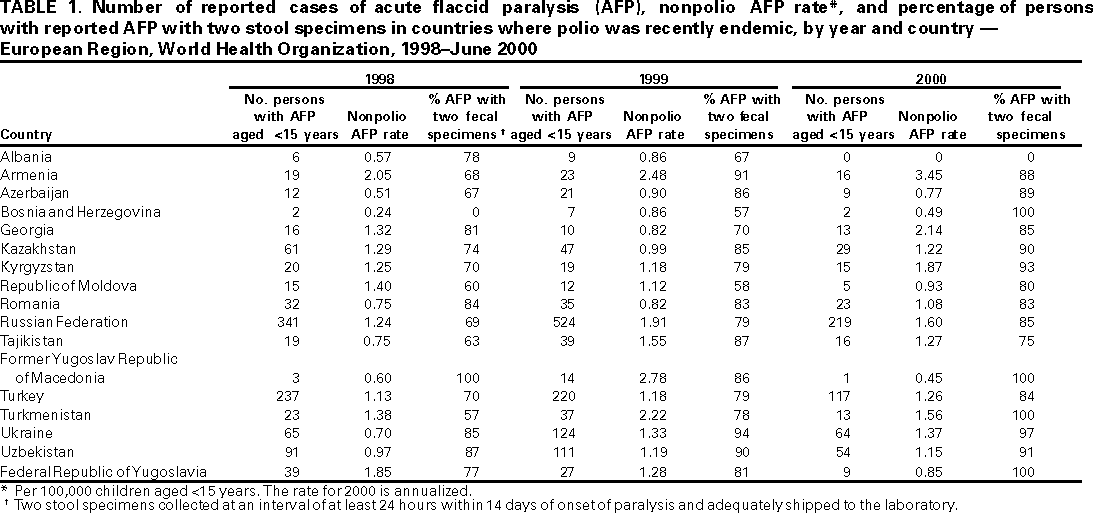 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 7/27/2000 |
|||||||||
This page last reviewed 5/2/01
|