Two-step Laboratory Testing Process
CDC currently recommends a two-step process when testing blood for evidence of antibodies against the Lyme disease bacteria. Both steps can be done using the same blood sample.
The first step uses a testing procedure called “EIA” (enzyme immunoassay) or rarely, an “IFA” (indirect immunofluorescence assay). If this first step is negative, no further testing of the specimen is recommended. If the first step is positive or indeterminate (sometimes called "equivocal"), the second step should be performed. The second step uses a test called an immunoblot test, commonly, a “Western blot” test. Results are considered positive only if the EIA/IFA and the immunoblot are both positive.
The two steps of Lyme disease testing are designed to be done together. CDC does not recommend skipping the first test and just doing the Western blot. Doing so will increase the frequency of false positive results and may lead to misdiagnosis and improper treatment.
New tests may be developed as alternatives to one or both steps of the two-step process. Before CDC will recommend new tests, their performance must be demonstrated to be equal to or better than the results of the existing procedure, and they must be FDA approved. For more details, see: Recommendations for Test Performance and Interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease.
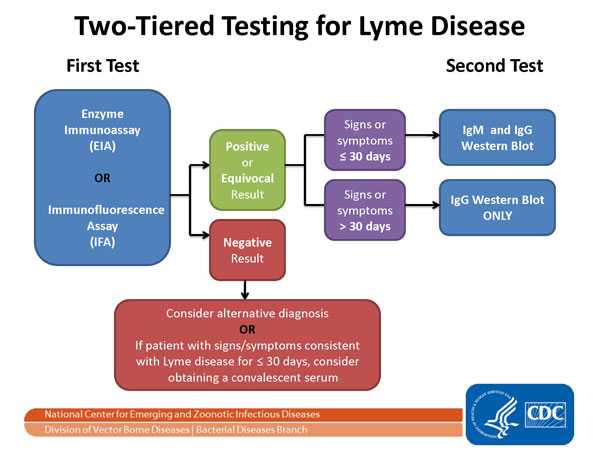
About This Figure
The Two-tier Testing Decision Tree describes the steps required to properly test for Lyme disease. The first required test is the Enzyme Immunoassay (EIA) or Immunofluorescence Assay (IFA). If this test yields negative results, the provider should consider an alternative diagnosis. Or in cases where the patient has had symptoms for less than or equal to 30 days, the provider may treat the patient and follow up with a convalescent serum. If the first test yields positive or equivocal results, two options are available: 1) if the patient has had symptoms for less than or equal to 30 days, an IgM Western Blot is performed; 2) if the patient has had symptoms for more than 30 days, the IgG Western Blot is performed. The IgM should not be used if the patient has been ill for more than 30 days.
- Page last reviewed: March 4, 2015
- Page last updated: March 26, 2015
- Content source:


 ShareCompartir
ShareCompartir