Guidelines for Environmental Infection Control in Health-Care Facilities (2003)
Background C. Air
On this Page
- Modes of Transmission of Airborne Diseases
-
Airborne Infectious Diseases
- Aspergillosis and Other Fungal Diseases
- Table 1. Clinical and epidemiologic characteristics of aspergillosis
- Table 2. Environmental fungal pathogens
- TB and Other Bacterial Diseases
- Table 3. Clinical and epidemiologic characteristics of TB
- Airborne Viral Diseases
- Table 4. Microorganisms associated with airborne transmission
-
Heating, Ventilation, and Air Conditioning Systems
- Basic Components and Operations
- Figure 1. Diagram of a ventilation system
- Filtration
- Table 5. Filtration methods
- Ultraviolet Germicidal Irradiation
- Conditioned Air in Occupied Spaces
- Table 6. Engineered specifications for positive- and negative pressure rooms
- Infection Control Impact of HVAC System Maintenance and Repair
-
Construction, Renovation, Remediation, Repair, and Demolition
- General Information
- Table 7. Ventilation hazards that may be associated with increased potential of airborne disease transmission
- Box 4. Suggested members and functions of a multi-disciplinary coordination team for construction
- Preliminary Considerations
- Box 5. Construction design and function considerations for environmental infection control
- Infection-Control Risk Assessment
- Air Sampling
- Box 6. Unresolved issues associated with microbiologic air sampling
- External Demolition and Construction
- Table 8. Strategies to reduce dust and moisture intrusion during external demolition and construction
- Internal Demolition, Construction, Renovations, and Repairs
- Box 7. Construction/repair projects that require barrier structures
- Table 9. Infection-control measures for internal construction
-
Environmental Infection-Control Measures for Special Settings
- Protective Environments
- Figure 2. Example of positive-pressure room control for protection from airborne environmental microbes
- Airborne Infection Isolation
- Figure 3. Example of negative-pressure room control for AII
- Figure 4. Example of AII room with anteroom and neutral anteroom
- Operating Rooms
- Box 8. Strategy for managing TB patients and preventing airborne transmission in operating room
- Table 10. Summary of ventilation specifications in selected areas
- Other Aerosol Hazards
1. Modes of Transmission of Airborne Diseases
A variety of airborne infections in susceptible hosts can result from exposures to clinically significant microorganisms released into the air when environmental reservoirs (i.e., soil, water, dust, and decaying organic matter) are disturbed. Once these materials are brought indoors into a health-care facility by any of a number of vehicles (e.g., people, air currents, water, construction materials, and equipment), the attendant microorganisms can proliferate in various indoor ecological niches and, if subsequently disbursed into the air, serve as a source for airborne health-care associated infections.
Respiratory infections can be acquired from exposure to pathogens contained either in droplets or droplet nuclei. Exposure to microorganisms in droplets (e.g., through aerosolized oral and nasal secretions from infected patients33 ) constitutes a form of direct contact transmission. When droplets are produced during a sneeze or cough, a cloud of infectious particles >5 μm in size is expelled, resulting in the potential exposure of susceptible persons within 3 feet of the source person.6 Examples of pathogens spread in this manner are influenza virus, rhinoviruses, adenoviruses, and respiratory syncytial virus (RSV). Because these agents primarily are transmitted directly and because the droplets tend to fall out of the air quickly, measures to control air flow in a health-care facility (e.g., use of negative pressure rooms) generally are not indicated for preventing the spread of diseases caused by these agents. Strategies to control the spread of these diseases are outlined in another guideline.3
The spread of airborne infectious diseases via droplet nuclei is a form of indirect transmission.34 Droplet nuclei are the residuals of droplets that, when suspended in air, subsequently dry and produce particles ranging in size from 1–5 μm. These particles can
- contain potentially viable microorganisms,
- be protected by a coat of dry secretions,
- remain suspended indefinitely in air, and
- be transported over long distances.
The microorganisms in droplet nuclei persist in favorable conditions (e.g., a dry, cool atmosphere with little or no direct exposure to sunlight or other sources of radiation). Pathogenic microorganisms that can be spread via droplet nuclei include Mycobacterium tuberculosis, VZV, measles virus (i.e., rubeola), and smallpox virus (i.e., variola major).6 Several environmental pathogens have life-cycle forms that are similar in size to droplet nuclei and may exhibit similar behavior in the air. The spores of Aspergillus fumigatus have a diameter of 2–3.5 μm, with a settling velocity estimated at 0.03 cm/second (or about 1 meter/hour) in still air. With this enhanced buoyancy, the spores, which resist desiccation, can remain airborne indefinitely in air currents and travel far from their source.35
2. Airborne Infectious Diseases in Health-Care Facilities
a. Aspergillosis and Other Fungal Diseases
Aspergillosis is caused by molds belonging to the genus Aspergillus. Aspergillus spp. are prototype health-care acquired pathogens associated with dusty or moist environmental conditions. Clinical and epidemiologic aspects of aspergillosis (Table 1) are discussed extensively in another guideline.3
Format Change [February 2017]
 The format of this section was changed to improve readability and accessibility. The content is unchanged.
The format of this section was changed to improve readability and accessibility. The content is unchanged.
Table 1. Clinical and epidemiologic characteristics of aspergillosis
Modes of transmission
Airborne transmission of fungal spores; direct inhalation; direct inoculation from environmental sources (rare) 37
Table 8. Strategies to reduce dust and moisture intrusion
Causative agents
Aspergillus fumigatus (90%–95% of Aspergillus infections among hematopoietic stem cell transplant (HSCT) patients; A. flavus, A. niger, A. terreus, A. nidulans 36–43
Activities associated with infection
Construction, renovation, remodeling, repairs, building demolition; rare episodes associated with fomites 44–51
Clinical syndromes and diseases
Acute invasive: pneumonia; ulcerative tracheobronchitis; osteomyelitis; abscesses (aspergillomas) of the lungs, brain, liver, spleen, and kidneys; thrombosis of deep blood vessels; necrotizing skin ulcers; endophthalmitis; and sinusitis Chronic invasive: chronic pneumonitis Hypersensity: allergic bronchopulmonary aspergillosis Cutaneous: primary skin and burn-wound infections 44, 45, 52–58
Patient populations at greatest risk
Hematopoietic stem cell transplant patients (HSCT): immunocompromised patients (ie, those with underlying disease), patients undergoing chemotherapy, organ transplant recipients, preterm neonates, hemodialysis patients, patients with identifiable immune system deficiencies who receive care in general intensive care units (ICUs), and cystic fibrosis patients (may be colonized, occasionally become infected) 3 6, 59–78
Factors affecting severity and outcomes
The immune status of the patient and the duration of severe neutropenia 79, 80
Occurrence
Rare and sporadic, but increasing as proportion of immunocompromised patients increases; 5% of HSCT patients infected, <5% of solid organ transplant recipients infected 36, 37, 81–88
Mortality rate
Rate can be as high as 100% if severe neutropenia persists; 13%–80% mortality among leukemia patients 5, 8, 83, 89, 90
Aspergillus spp. are ubiquitous, aerobic fungi that occur in soil, water, and decaying vegetation; the organism also survives well in air, dust, and moisture present in health-care facilities.91–93 The presence of aspergilli in the health-care facility environment is a substantial extrinsic risk factor for opportunistic invasive aspergillosis (invasive aspergillosis being the most serious form of the disease).69, 94 Site renovation and construction can disturb Aspergillus-contaminated dust and produce bursts of airborne fungal spores. Increased levels of atmospheric dust and fungal spores have been associated with clusters of health-care acquired infections in immunocompromised patients.17, 20, 44, 47, 49, 50, 95–98 Absorbent building materials (e.g., wallboard) serve as an ideal substrate for the proliferation of this organism if they become and remain wet, thereby increasing the numbers of fungal spores in the area. Patient-care items, devices, and equipment can become contaminated with Aspergillus spp. spores and serve as sources of infection if stored in such areas.57
Most cases of aspergillosis are caused by Aspergillus fumigatus, a thermotolerant/thermophilic fungus capable of growing over a temperature range from 53.6°F–127.4°F (12°C–53°C); optimal growth occurs at approximately 104°F (40°C), a temperature inhibitory to most other saprophytic fungi.99 It can use cellulose or sugars as carbon sources; because its respiratory process requires an ample supply of carbon, decomposing organic matter is an ideal substrate. (For AIA guidance on Aspergillus spp. see Table 2.)
Other opportunistic fungi that have been occasionally linked with health-care associated infections are members of the order Mucorales (e.g., Rhizopus spp.) and miscellaneous moniliaceous molds (e.g., Fusarium spp. and Penicillium spp.) (Table 2). Many of these fungi can proliferate in moist environments (e.g., water-damaged wood and building materials). Some fungi (e.g., Fusarium spp. and Pseudoallescheria spp.) also can be airborne pathogens.100 As with aspergillosis, a major risk factor for disease caused by any of these pathogens is the host’s severe immunosuppression from either underlying disease or immunosuppressive therapy.101, 102
Format Change [February 2017]
 The format of this section was changed to improve readability and accessibility. The content is unchanged.
The format of this section was changed to improve readability and accessibility. The content is unchanged.
Table 2. Environmental fungal pathogens: entry into and contamination of the healthcare facility
| Fungal pathogen | Implicated environmental vehicle |
|---|---|
| Aspergillus spp. |
|
| Mucorales / Rhizopus spp. |
|
| Scedosporium spp. |
|
| Penicillium spp. |
|
| Acremonium spp. |
|
| Cladosporium spp. |
|
| Sporothrix |
|
+ The American Institute of Architects (AIA) standards stipulate that for new or renovated construction
- exhaust outlets are to be placed >25 feet from air intake systems,
- the bottom of outdoor air intakes for HVAC systems should be 6 feet above ground or 3 feet above roof level, and
- exhaust outlets from contaminated areas are situated above the roof level and arranged to minimize the recirculation of exhausted air back into the building.120
Infections due Cryptococcus neoformans, Histoplasma capsulatum, or Coccidioides immitis can occur in health-care settings if nearby ground is disturbed and a malfunction of the facility’s air-intake components allows these pathogens to enter the ventilation system. C. neoformans is a yeast usually 4– 8 μm in size. However, viable particles of <2 μm diameter (and thus permissive to alveolar deposition) have been found in soil contaminated with bird droppings, particularly from pigeons.98, 103, 104, 121 H. capsulatum, with the infectious microconidia ranging in size from 2–5 μm, is endemic in the soil of the central river valleys of the United States. Substantial numbers of these infectious particles have been associated with chicken coops and the roosts of blackbirds.98, 103, 104, 122 Several outbreaks of histoplasmosis have been associated with disruption of the environment; construction activities in an endemic area may be a potential risk factor for health-care acquired airborne infection.123, 124 C. immitis, with arthrospores of 3–5 μm diameter, has similar potential, especially in the endemic southwestern United States and during seasons of drought followed by heavy rainfall. After the 1994 earthquake centered near Northridge, California, the incidence of coccidioidomycosis in the surrounding area exceeded the historical norm.125
Emerging evidence suggests that Pneumocystis carinii, now classified as a fungus, may be spread via airborne, person-to-person transmission.126 Controlled studies in animals first demonstrated that P. carinii could be spread through the air.127 More recent studies in health-care settings have detected nucleic acids of P. carinii in air samples from areas frequented or occupied by P. carinii-infected patients but not in control areas that are not occupied by these patients.128, 129 Clusters of cases have been identified among immunocompromised patients who had contact with a source patient and with each other. Recent studies have examined the presence of P. carinii DNA in oropharyngeal washings and the nares of infected patients, their direct contacts, and persons with no direct contact.130, 131 Molecular analysis of the DNA by polymerase chain reaction (PCR) provides evidence for airborne transmission of P. carinii from infected patients to direct contacts, but immunocompetent contacts tend to become transiently colonized rather than infected.131 The role of colonized persons in the spread of P. carinii pneumonia (PCP) remains to be determined. At present, specific modifications to ventilation systems to control spread of PCP in a health-care facility are not indicated. Current recommendations outline isolation procedures to minimize or eliminate contact of immunocompromised patients not on PCP prophylaxis with PCP-infected patients.6, 132
b. Tuberculosis and Other Bacterial Diseases
The bacterium most commonly associated with airborne transmission is Mycobacterium tuberculosis. A comprehensive review of the microbiology and epidemiology of M. tuberculosis and guidelines for tuberculosis (TB) infection control have been published.4, 133, 134 A summary of the clinical and epidemiologic information from these materials is provided in this guideline (Table 3).
Format Change [February 2017]
 The format of this section was changed to improve readability and accessibility. The content is unchanged.
The format of this section was changed to improve readability and accessibility. The content is unchanged.
Table 3. Clinical and epidemiologic characteristics of tuberculosis (TB)*
Modes of transmission
Airborne transmission via droplet nuclei 1–5 μm in diameter
Causative agents
Mycobacterium tuberculosis, M. bovis, M. africanum
Patient factors associated with infectivity and transmission
- Disease of the lungs, airways, or larynx
- Presence of cough or other forceful expiratory measures
- Presence of acid-fast bacilli (AFB) in the sputum
- Failure of the patient to cover the mouth and nose when coughing or sneezing
- Presence of cavitation on chest radiograph
- Inappropriate or shortened duration of chemotherapy
Activities associated with infections
- Exposures in relatively small, enclosed spaces
- Inadequate ventilation resulting in insufficient removal of droplet nuclei
- Cough-producing procedures done in areas without proper environmental controls
- Recirculation of air containing infectious droplet nuclei
- Failure to use respiratory protection when managing open lesions for patients with suspected extrapulmonary TB135
Clinical syndromes and disease
- Pulmonary TB
- Extrapulmonary TB can affect any organ system or tissue
- Laryngeal TB is highly contagious
Patient populations at greatest risk
- Immunocompromised persons (eg, HIV-infected persons)
- Medically underserved persons, urban poor, homeless persons, elderly persons, migrant farm workers, close contacts of known patients
- Substance abusers, present and former prison inmates
- Foreign-born persons from areas with high prevalence of TB
- Health-care workers
Factors affecting severity and outcomes
- Concentration of droplet nuclei in air, duration of exposure
- Age at infection
- Immunosuppression due to therapy or disease, underlying chronic medical conditions, history of malignancies or lesions or the lungs
Occurrence
- Worldwide; incidence in the United States is 56 cases/100,000 population (2001)136
Mortality rate
- 930 deaths in the United States (1999)136
Chemoprophylaxis / treatment
- Treatment of latent infection includes isoniazid (INH) or rifampin (RIF)4, 134, 137–139
- Directly observed therapy (DOT) for active cases as indicated: INH, RIF, pyrazinamide (PZA), ethambutol (EMB), streptomycin (SM) in various combinations determined by prevalent levels of specific resistance4, 134, 137–139
- Consult therapy guidelines for specific treatment indications139
* Material in this table is compiled from references 4, 133–141.
M. tuberculosis is carried by droplet nuclei generated when persons (primarily adults and adolescents) who have pulmonary or laryngeal TB sneeze, cough, speak, or sing;139 normal air currents can keep these particles airborne for prolonged periods and spread them throughout a room or building.142 However, transmission of TB has occurred from mycobacteria aerosolized during provision of care (e.g., wound/lesion care or during handling of infectious peritoneal dialysis fluid) for extrapulmonary TB patients.135, 140
Gram-positive cocci (i.e., Staphylococcus aureus, group A beta-hemolytic streptococci), also important health-care associated pathogens, are resistant to inactivation by drying and can persist in the environment and on environmental surfaces for extended periods. These organisms can be shed from heavily colonized persons and discharged into the air. Airborne dispersal of S. aureus is directly associated with the concentration of the bacterium in the anterior nares.143 Approximately 10% of healthy carriers will disseminate S. aureus into the air, and some persons become more effective disseminators of S. aureus than others.144–148 The dispersal of S. aureus into air can be exacerbated by concurrent viral upper respiratory infection, thereby turning a carrier into a “cloud shedder.”149 Outbreaks of surgical site infections (SSIs) caused by group A beta-hemolytic streptococci have been traced to airborne transmission from colonized operating-room personnel to patients.150–153 In these situations, the strain causing the outbreak was recovered from the air in the operating room150, 151, 154 or on settle plates in a room in which the carrier exercised.151–153 S. aureus and group A streptococci have not been linked to airborne transmission outside of operating rooms, burn units, and neonatal nurseries.155, 156 Transmission of these agents occurs primarily via contact and droplets.
Other gram-positive bacteria linked to airborne transmission include Bacillus spp. which are capable of sporulation as environmental conditions become less favorable to support their growth. Outbreaks and pseudo-outbreaks have been attributed to Bacillus cereus in maternity, pediatric, intensive care, and bronchoscopy units; many of these episodes were secondary to environmental contamination.157–160
Gram-negative bacteria rarely are associated with episodes of airborne transmission because they generally require moist environments for persistence and growth. The main exception is Acinetobacter spp., which can withstand the inactivating effects of drying. In one epidemiologic investigation of bloodstream infections among pediatric patients, identical Acinetobacter spp. were cultured from the patients, air, and room air conditioners in a nursery.161
Aerosols generated from showers and faucets may potentially contain legionellae and other gram-negative waterborne bacteria (e.g., Pseudomonas aeruginosa). Exposure to these organisms is through direct inhalation. However, because water is the source of the organisms and exposure occurs in the vicinity of the aerosol, the discussion of the diseases associated with such aerosols and the prevention measures used to curtail their spread is discussed in another section of the Guideline (see Part I: Water).
c. Airborne Viral Diseases
Some human viruses are transmitted from person to person via droplet aerosols, but very few viruses are consistently airborne in transmission (i.e., are routinely suspended in an infective state in air and capable of spreading great distances), and health-care associated outbreaks of airborne viral disease are limited to a few agents. Consequently, infection-control measures used to prevent spread of these viral diseases in health-care facilities primarily involve patient isolation, vaccination of susceptible persons, and antiviral therapy as appropriate rather than measures to control air flow or quality.6 Infections caused by VZV frequently are described in health-care facilities. Health-care associated airborne outbreaks of VZV infections from patients with primary infection and disseminated zoster have been documented; patients with localized zoster have, on rare occasions, also served as source patients for outbreaks in health-care facilities.162–166 VZV infection can be prevented by vaccination, although patients who develop a rash within 6 weeks of receiving varicella vaccine or who develop breakthrough varicella following exposure should be considered contagious.167
Viruses whose major mode of transmission is via droplet contact rarely have caused clusters of infections in group settings through airborne routes. The factors facilitating airborne distribution of these viruses in an infective state are unknown, but a presumed requirement is a source patient in the early stage of infection who is shedding large numbers of viral particles into the air. Airborne transmission of measles has been documented in health-care facilities.168–171 In addition, institutional outbreaks of influenza virus infections have occurred predominantly in nursing homes,172–176 and less frequently in medical and neonatal intensive care units, chronic-care areas, HSCT units, and pediatric wards.177–180 Some evidence supports airborne transmission of influenza viruses by droplet nuclei,181, 182 and case clusters in pediatric wards suggest that droplet nuclei may play a role in transmitting certain respiratory pathogens (e.g., adenoviruses and respiratory syncytial virus [RSV]).177, 183, 184 Some evidence also supports airborne transmission of enteric viruses. An outbreak of a Norwalk-like virus infection involving more than 600 staff personnel over a 3-week period was investigated in a Toronto, Ontario hospital in 1985; common sources (e.g., food and water) were ruled out during the investigation, leaving airborne spread as the most likely mode of transmission.185
Smallpox virus, a potential agent of bioterrorism, is spread predominantly via direct contact with infectious droplets, but it also can be associated with airborne transmission.186, 187 A German hospital study from 1970 documented the ability of this virus to spread over considerable distances and cause infection at low doses in a well-vaccinated population; factors potentially facilitating transmission in this situation included a patient with cough and an extensive rash, indoor air with low relative humidity, and faulty ventilation patterns resulting from hospital design (e.g., open windows).188 Smallpox patients with extensive rash are more likely to have lesions present on mucous membranes and therefore have greater potential to disseminate virus into the air.188 In addition to the smallpox transmission in Germany, two cases of laboratory-acquired smallpox virus infection in the United Kingdom in 1978 also were thought to be caused by airborne transmission.189
Ebola Virus Disease [August 2014]
 Update: The recommendations in this guideline for Ebola has been superseded by these CDC documents:
Update: The recommendations in this guideline for Ebola has been superseded by these CDC documents:
- Infection Prevention and Control Recommendations for Hospitalized Patients with Known or Suspected Ebola Virus Disease in U.S. Hospitals
- Interim Guidance for Environmental Infection Control in Hospitals for Ebola Virus
See CDC’s Ebola Virus Disease website for current information on how Ebola virus is transmitted.
Airborne transmission may play a role in the natural spread of hantaviruses and certain hemorrhagic fever viruses (e.g., Ebola, Marburg, and Lassa), but evidence for airborne spread of these agents in health-care facilities is inconclusive.190 Although hantaviruses can be transmitted when aerosolized from rodent excreta,191, 192 person-to-person spread of hantavirus infection from source patients has not occurred in health-care facilities.193–195 Nevertheless, health-care workers are advised to contain potentially infectious aerosols and wear National Institute of Occupational Safety and Health (NIOSH) approved respiratory protection when working with this agent in laboratories or autopsy suites.196 Lassa virus transmission via aerosols has been demonstrated in the laboratory and incriminated in health-care associated infections in Africa,197–199 but airborne spread of this agent in hospitals in developed nations likely is inefficient.200, 201 Yellow fever is considered to be a viral hemorrhagic fever agent with high aerosol infectivity potential, but health-care associated transmission of this virus has not been described.202 Viral hemorrhagic fever diseases primarily occur after direct exposure to infected blood and body fluids, and the use of standard and droplet precautions prevents transmission early in the course of these illnesses.203, 204 However, whether these viruses can persist in droplet nuclei that might remain after droplet production from coughs or vomiting in the latter stages of illness is unknown.205 Although the use of a negative-pressure room is not required during the early stages of illness, its use might be prudent at the time of hospitalization to avoid the need for subsequent patient transfer. Current CDC guidelines recommend negative-pressure rooms with anterooms for patients with hemorrhagic fever and use of HEPA respirators by persons entering these rooms when the patient has prominent cough, vomiting, diarrhea, or hemorrhage.6, 203 Face shields or goggles will help to prevent mucous-membrane exposure to potentially-aerosolized infectious material in these situations. If an anteroom is not available, portable, industrial-grade high efficiency particulate air (HEPA) filter units can be used to provide the equivalent of additional air changes per hour (ACH).
Table 4. Microorganisms associated with airborne transmission*
| Evidence for airborne transmission | Fungi | Bacteria | Viruses |
|---|---|---|---|
| Numerous reports in health-care facilities | Aspergillus spp.+ Mucorales (Rhizopus spp.)97, 115 |
Mycobacterium tuberculosis+ | Measles (rubeola) virus168-170 Varicella-zoster virus162-166 |
| Occasional reports in health-care facilities (atypical) | Acremonium spp.105, 206 Fusarium spp.102 Pseudoallescheria boydii100 Scedosporium spp.116 Sporothrix cyanescens¶118 |
Acinetobacter spp.161 Bacillus spp.¶160, 207 Brucella spp.**208-211 Staphylococcus aureus148, 156 Group A Streptococcus151 |
Smallpox virus (variola)§188, 189 Influenza viruses181, 182 Respiratory syncytial virus183 Adenoviruses184 Norwalk-like virus185 |
| No reports in health-care facilities; known to be airborne outside. | Coccidioides immitis125 Cryptococcus spp.121 Histoplasma capsulatum124 |
Coxiella burnetii (Q fever)212 | Hantaviruses193, 195 Lassa virus205 Marburg virus205 Ebola virus†205 Crimean-Congo virus205 |
| Under investigation | Pneumocystis carinii131 | n/a | n/a |
* This list excludes microorganisms transmitted from aerosols derived from water.
+ Refer to the text for references for these disease agents.
§ Airborne transmission of smallpox is infrequent. Potential for airborne transmission increases with patients who are effective disseminators present in facilities with low relative humidity in the air and faulty ventilation.
¶ Documentation of pseudoepidemic during construction.
** Airborne transmission documented in the laboratory but not in patient-care areas.
† The recommendations in this guideline for Ebola Virus Disease has been superseded on August 1, 2014.
3. Heating, Ventilation, and Air Conditioning Systems in Health-Care Facilities
a. Basic Components and Operations
Heating, ventilation, and air conditioning (HVAC) systems in health-care facilities are designed to
- maintain the indoor air temperature and humidity at comfortable levels for staff, patients, and visitors
- control odors;
- remove contaminated air;
- facilitate air-handling requirements to protect susceptible staff and patients from airborne health-care associated pathogens; and
- minimize the risk for transmission of airborne pathogens from infected patients.35, 120
An HVAC system includes an outside air inlet or intake; filters; humidity modification mechanisms (i.e., humidity control in summer, humidification in winter); heating and cooling equipment; fans; ductwork; air exhaust or out-takes; and registers, diffusers, or grilles for proper distribution of the air (Figure 1).213, 214 Decreased performance of healthcare facility HVAC systems, filter inefficiencies, improper installation, and poor maintenance can contribute to the spread of health-care associated airborne infections.
The American Institute of Architects (AIA) has published guidelines for the design and construction of new health-care facilities and for renovation of existing facilities. These AIA guidelines address indoor air-quality standards (e.g., ventilation rates, temperature levels, humidity levels, pressure relationships, and minimum air changes per hour [ACH]) specific to each zone or area in health-care facilities (e.g., operating rooms, laboratories, diagnostic areas, patient-care areas, and support departments).120 These guidelines represent a consensus document among authorities having jurisdiction (AHJ), governmental regulatory agencies (i.e., Department of Health and Human Services [DHHS]; Department of Labor, Occupational Safety and Health Administration [OSHA]), health-care professionals, professional organizations (e.g., American Society of Heating, Refrigeration, and Air-Conditioning Engineers [ASHRAE], American Society for Healthcare Engineering [ASHE]), and accrediting organizations (i.e., Joint Commission on Accreditation of Healthcare Organizations [JCAHO]). More than 40 state agencies that license health-care facilities have either incorporated or adopted by reference these guidelines into their state standards. JCAHO, through its surveys, ensures that facilities are in compliance with the ventilation guidelines of this standard for new construction and renovation.
Figure 1. Diagram of a ventilation system*
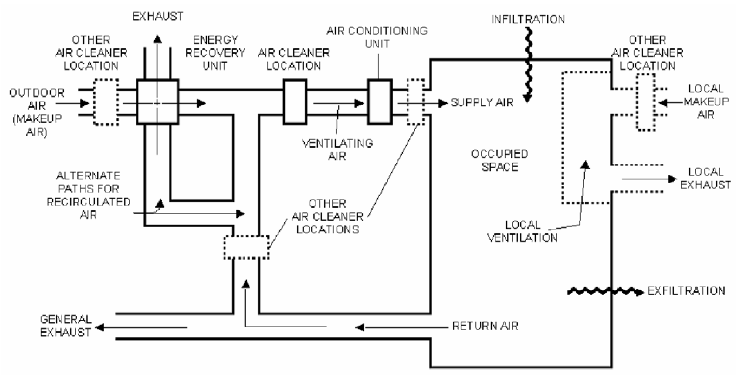
Outdoor air and recirculated air pass through air cleaners (e.g., filter banks) designed to reduce the concentration of airborne contaminants. Air is conditioned for temperature and humidity before it enters the occupied space as supply air. Infiltration is air leakage inward through cracks and interstitial spaces of walls, floors, and ceilings. Exfiltration is air leakage outward through these same cracks and spaces. Return air is largely exhausted from the system, but a portion is recirculated with fresh, incoming air.
* Used with permission of the publisher of reference 214 (ASHRAE)
Engineering controls to contain or prevent the spread of airborne contaminants center on
- local exhaust ventilation [i.e., source control],
- general ventilation, and
- air cleaning.4
General ventilation encompasses
- dilution and removal of contaminants via well-mixed air distribution of filtered air,
- directing contaminants toward exhaust registers and grilles via uniform, non-mixed airflow patterns,
- pressurization of individual spaces relative to all other spaces, and
- pressurization of buildings relative to the outdoors and other attached buildings.
A centralized HVAC system operates as follows. Outdoor air enters the system, where low-efficiency or “roughing” filters remove large particulate matter and many microorganisms. The air enters the distribution system for conditioning to appropriate temperature and humidity levels, passes through an additional bank of filters for further cleaning, and is delivered to each zone of the building. After the conditioned air is distributed to the designated space, it is withdrawn through a return duct system and delivered back to the HVAC unit. A portion of this “return air” is exhausted to the outside while the remainder is mixed with outdoor air for dilution and filtered for removal of contaminants.215 Air from toilet rooms or other soiled areas is usually exhausted directly to the atmosphere through a separate duct exhaust system. Air from rooms housing tuberculosis patients is exhausted to the outside if possible, or passed through a HEPA filter before recirculation. Ultraviolet germicidal irradiation (UVGI) can be used as an adjunct air-cleaning measure, but it cannot replace HEPA filtration. 15
b. Filtration
i. Filter Types and Methods of Filtration
Filtration, the physical removal of particulates from air, is the first step in achieving acceptable indoor air quality. Filtration is the primary means of cleaning the air. Five methods of filtration can be used (Table 5). During filtration, outdoor air passes through two filter beds or banks (with efficiencies of 20%–40% and ≥90%, respectively) for effective removal of particles 1–5 μm in diameter.35, 120 The low-to-medium efficiency filters in the first bank have low resistance to airflow, but this feature allows some small particulates to pass onto heating and air conditioning coils and into the indoor environment.35 Incoming air is mixed with recirculated air and reconditioned for temperature and humidity before being filtered by the second bank of filters. The performance of filters with ≤90% efficiency is measured using either the dust-spot test or the weight-arrestance test.35, 216
Table 5. Filtration methods*
| Basic method | Principle of performance | Filtering efficiency |
|---|---|---|
| Straining | Particles in the air are larger than the openings between the filter fibers, resulting in gross removal of large particles. | Low |
| Impingement | Particles collide with filter fibers and remain attached to the filter. Fibers may be coated with adhesive. | Low |
| Interception | Particles enter into the filter and become entrapped and attached to the filter fibers. | Medium |
| Diffusion | Small particles, moving in erratic motion, collide with filter fibers and remain attached. | High |
| Electrostatic | Particles bearing negative electrostatic charge are attracted to the filter with positively charged fibers. | High |
* Material in this table was compiled from information in reference 217.
The second filter bank usually consists of high-efficiency filters. This filtration system is adequate for most patient-care areas in ambulatory-care facilities and hospitals, including the operating room environment and areas providing central services.120 Nursing facilities use 90% dust-spot efficient filters as the second bank of filters,120 whereas a HEPA filter bank may be indicated for special-care areas of hospitals. HEPA filters are at least 99.97% efficient for removing particles ≥0.3 μm in diameter. (As a reference, Aspergillus spores are 2.5–3.0 μm in diameter.) Examples of care areas where HEPA filters are used include PE rooms and those operating rooms designated for orthopedic implant procedures.35
Maintenance costs associated with HEPA filters are high compared with other types of filters, but use of in-line disposable prefilters can increase the life of a HEPA filter by approximately 25%. Alternatively, if a disposable prefilter is followed by a filter that is 90% efficient, the life of the HEPA filter can be extended ninefold. This concept, called progressive filtration, allows HEPA filters in special care areas to be used for 10 years.213 Although progressive filtering will extend the mechanical ability of the HEPA filter, these filters may absorb chemicals in the environment and later desorb those chemicals, thereby necessitating a more frequent replacement program. HEPA filter efficiency is monitored with the dioctylphthalate (DOP) particle test using particles that are 0.3 μm in diameter.218
HEPA filters are usually framed with metal, although some older versions have wood frames. A metal frame has no advantage over a properly fitted wood frame with respect to performance, but wood can compromise the air quality if it becomes and remains wet, allowing the growth of fungi and bacteria. Hospitals are therefore advised to phase out water-damaged or spent wood-framed filter units and replace them with metal-framed HEPA filters.
HEPA filters are usually fixed into the HVAC system; however, portable, industrial grade HEPA units are available that can filter air at the rate of 300–800 ft3 /min. Portable HEPA filters are used to
- temporarily recirculate air in rooms with no general ventilation,
- augment systems that cannot provide adequate airflow, and
- provide increased effectiveness in airflow.4
Portable HEPA units are useful engineering controls that help clean the air when the central HVAC system is undergoing repairs219 but these units do not satisfy fresh-air requirements.214
The effectiveness of the portable unit for particle removal is dependent on
- the configuration of the room,
- the furniture and persons in the room,
- the placement of the units relative to the contents and layout of the room, and
- the location of the supply and exhaust registers or grilles.
If portable, industrial-grade units are used, they should be capable of recirculating all or nearly all of the room air through the HEPA filter, and the unit should be designed to achieve the equivalent of ≥12 ACH.4 (An average room has approximately 1,600 ft3 of airspace.) The hospital engineering department should be contacted to provide ACH information in the event that a portable HEPA filter unit is necessary to augment the existing fixed HVAC system for air cleaning.
ii. Filter Maintenance
Efficiency of the filtration system is dependent on the density of the filters, which can create a drop in pressure unless compensated by stronger and more efficient fans, thus maintaining air flow. For optimal performance, filters require monitoring and replacement in accordance with the manufacturer’s recommendations and standard preventive maintenance practices.220 Upon removal, spent filters can be bagged and discarded with the routine solid waste, regardless of their patient-care area location.221 Excess accumulation of dust and particulates increases filter efficiency, requiring more pressure to push the air through. The pressure differential across filters is measured by use of manometers or other gauges. A pressure reading that exceeds specifications indicates the need to change the filter. Filters also require regular inspection for other potential causes of decreased performance. Gaps in and around filter banks and heavy soil and debris upstream of poorly maintained filters have been implicated in health-care associated outbreaks of aspergillosis, especially when accompanied by construction activities at the facility.17, 18, 106, 222
c. Ultraviolet Germicidal Irradiation (UVGI)
As a supplemental air-cleaning measure, UVGI is effective in reducing the transmission of airborne bacterial and viral infections in hospitals, military housing, and classrooms, but it has only a minimal inactivating effect on fungal spores.223–228 UVGI is also used in air handling units to prevent or limit the growth of vegetative bacteria and fungi. Most commercially available UV lamps used for germicidal purposes are low-pressure mercury vapor lamps that emit radiant energy predominantly at a wave-length of 253.7 nm.229, 230 Two systems of UVGI have been used in health-care settings – duct irradiation and upper-room air irradiation. In duct irradiation systems, UV lamps are placed inside ducts that remove air from rooms to disinfect the air before it is recirculated. When properly designed, installed, and maintained, high levels of UVGI can be attained in the ducts with little or no exposure of persons in the rooms.231, 232 In upper-room air irradiation, UV lamps are either suspended from the ceiling or mounted on the wall.4 Upper air UVGI units have two basic designs:
- a “pan” fixture with UVGI unshielded above the unit to direct the irradiation upward and
- a fixture with a series of parallel plates to columnize the irradiation outward while preventing the light from getting to the eyes of the room’s occupants.
The germicidal effect is dependent on air mixing via convection between the room’s irradiated upper zone and the lower patient-care zones.233, 234
Bacterial inactivation studies using BCG mycobacteria and Serratia marcescens have estimated the effect of UVGI as equivalent to 10 ACH–39 ACH.235, 236 Another study, however, suggests that UVGI may result in fewer equivalent ACH in the patient-care zone, especially if the mixing of air between zones is insufficient.234 The use of fans or HVAC systems to generate air movement may increase the effectiveness of UVGI if airborne microorganisms are exposed to the light energy for a sufficient length of time.233, 235, 237–239 The optimal relationship between ventilation and UVGI is not known.
Because the clinical effectiveness of UV systems may vary, UVGI is not recommended for air management prior to air recirculation from airborne isolation rooms. It is also not recommended as a substitute for HEPA filtration, local exhaust of air to the outside, or negative pressure.4 The use of UV lamps and HEPA filtration in a single unit offers only minimal infection-control benefits over those provided by the use of a HEPA filter alone.240 Duct systems with UVGI are not recommended as a substitute for HEPA filters if the air from isolation rooms must be recirculated to other areas of the facility.4 Regular maintenance of UVGI systems is crucial and usually consists of keeping the bulbs free of dust and replacing old bulbs as necessary. Safety issues associated with the use of UVGI systems are described in other guidelines.4
d. Conditioned Air in Occupied Spaces
Temperature and humidity are two essential components of conditioned air. After outside air passes through a low- or medium-efficiency filter, the air undergoes conditioning for temperature and humidity control before it passes through high-efficiency or HEPA filtration.
i. Temperature
HVAC systems in health-care facilities are often single-duct or dual-duct systems.35, 241 A single-duct system distributes cooled air (55°F [12.8°C]) throughout the building and uses thermostatically controlled reheat boxes located in the terminal ductwork to warm the air for individual or multiple rooms. The dual-duct system consists of parallel ducts, one with a cold air stream and the other with a hot air stream. A mixing box in each room or group of rooms mixes the two air streams to achieve the desired temperature. Temperature standards are given as either a single temperature or a range, depending on the specific health-care zone. Cool temperature standards (68°F–73°F [20°C–23°C]) usually are associated with operating rooms, clean workrooms, and endoscopy suites.120 A warmer temperature (75°F [24°C]) is needed in areas requiring greater degrees of patient comfort. Most other zones use a temperature range of 70°F–75°F (21°C–24°C).120 Temperatures outside of these ranges may be needed occasionally in limited areas depending on individual circumstances during patient care (e.g., cooler temperatures in operating rooms during specialized operations).
ii. Humidity
Four measures of humidity are used to quantify different physical properties of the mixture of water vapor and air. The most common of these is relative humidity, which is the ratio of the amount of water vapor in the air to the amount of water vapor air can hold at that temperature.242 The other measures of humidity are specific humidity, dew point, and vapor pressure.242
Relative humidity measures the percentage of saturation. At 100% relative humidity, the air is saturated. For most areas within health-care facilities, the designated comfort range is 30%–60% relative humidity.120, 214 Relative humidity levels >60%, in addition to being perceived as uncomfortable, promote fungal growth.243 Humidity levels can be manipulated by either of two mechanisms.244 In a water-wash unit, water is sprayed and drops are taken up by the filtered air; additional heating or cooling of this air sets the humidity levels. The second mechanism is by means of water vapor created from steam and added to filtered air in humidifying boxes. Reservoir-type humidifiers are not allowed in health-care facilities as per AIA guidelines and many state codes.120 Cool-mist humidifiers should be avoided, because they can disseminate aerosols containing allergens and microorganisms.245 Additionally, the small, personal-use versions of this equipment can be difficult to clean.
iii. Ventilation
The control of air pollutants (e.g., microorganisms, dust, chemicals, and smoke) at the source is the most effective way to maintain clean air. The second most effective means of controlling indoor air pollution is through ventilation. Ventilation rates are voluntary unless a state or local government specifies a standard in health-care licensing or health department requirements. These standards typically apply to only the design of a facility, rather than its operation.220, 246 Health-care facilities without specific ventilation standards should follow the AIA guideline specific to the year in which the building was 120, 214, 241 built or the ANSI/ASHRAE Standard 62, Ventilation for Acceptable Indoor Air Quality.
Ventilation guidelines are defined in terms of air volume per minute per occupant and are based on the assumption that occupants and their activities are responsible for most of the contaminants in the conditioned space.215 Most ventilation rates for health-care facilities are expressed as room ACH. Peak efficiency for particle removal in the air space occurs between 12 ACH–15 ACH.35, 247, 248 Ventilation rates vary among the different patient-care areas of a health-care facility (Appendix B).120
Health-care facilities generally use recirculated air.35, 120, 241, 249, 250 Fans create sufficient positive pressure to force air through the building duct work and adequate negative pressure to evacuate air from the conditioned space into the return duct work and/or exhaust, thereby completing the circuit in a sealed system (Figure 1). However, because gaseous contaminants tend to accumulate as the air recirculates, a percentage of the recirculated air is exhausted to the outside and replaced by fresh outdoor air. In hospitals, the delivery of filtered air to an occupied space is an engineered system design issue, the full discussion of which is beyond the scope of this document.
Hospitals with areas not served by central HVAC systems often use through-the-wall or fan coil air conditioning units as the sole source of room ventilation. AIA guidelines for newly installed systems stipulate that through-the-wall fan-coil units be equipped with permanent (i.e., cleanable) or replaceable filters with a minimum efficiency of 68% weight arrestance.120 These units may be used only as recirculating units; all outdoor air requirements must be met by a separate central air handling system with proper filtration, with a minimum of two outside air changes in general patient rooms (D. Erickson, ASHE, 2000).120 If a patient room is equipped with an individual through-the-wall fan coil unit, the room should not be used as either AII or as PE.120 These requirements, although directed to new HVAC installations also are appropriate for existing settings. Non-central air-handling systems are prone to problems associated with excess condensation accumulating in drip pans and improper filter maintenance; health-care facilities should clean or replace the filters in these units on a regular basis while the patient is out of the room.
Laminar airflow ventilation systems are designed to move air in a single pass, usually through a bank of HEPA filters either along a wall or in the ceiling, in a one-way direction through a clean zone with parallel streamlines. Laminar airflow can be directed vertically or horizontally; the unidirectional system optimizes airflow and minimizes air turbulence.63, 241 Delivery of air at a rate of 0.5 meters per second (90 ± 20 ft/min) helps to minimize opportunities for microorganism proliferation.63, 251, 252 Laminar airflow systems have been used in PE to help reduce the risk for health-care associated airborne infections (e.g., aspergillosis) in high-risk patients.63, 93, 253, 254 However, data that demonstrate a survival benefit for patients in PE with laminar airflow are lacking. Given the high cost of installation and apparent lack of benefit, the value of laminar airflow in this setting is questionable.9, 37 Few data support the use of laminar airflow systems elsewhere in a hospital.255
iv. Pressurization
Positive and negative pressures refer to a pressure differential between two adjacent air spaces (e.g., rooms and hallways). Air flows away from areas or rooms with positive pressure (pressurized), while air flows into areas with negative pressure (depressurized). AII rooms are set at negative pressure to prevent airborne microorganisms in the room from entering hallways and corridors. PE rooms housing severely neutropenic patients are set at positive pressure to keep airborne pathogens in adjacent spaces or corridors from coming into and contaminating the airspace occupied by such high-risk patients. Self-closing doors are mandatory for both of these areas to help maintain the correct pressure differential.4, 6, 120 Older health-care facilities may have variable pressure rooms (i.e., rooms in which the ventilation can be manually switched between positive and negative pressure). These rooms are no longer permitted in the construction of new facilities or in renovated areas of the facility,120 and their use in existing facilities has been discouraged because of difficulties in assuring the proper pressure differential, especially for the negative pressure setting, and because of the potential for error associated with switching the pressure differentials for the room. Continued use of existing variable pressure rooms depends on a partnership between engineering and infection control. Both positive- and negative-pressure rooms should be maintained according to specific engineering specifications (Table 6).
Table 6. Engineered specifications for positive- and negative pressure rooms*
| Engineering characteristics | Positive pressure areas (e.g., protective environments [PE]) |
Negative pressure areas (e.g., airborne infection isolation [AII]) |
|---|---|---|
| Pressure differentials | > +2.5 Pa§ (0.01″ water gauge) | > −2.5 Pa (0.01″ water gauge) |
| Air changes per hour (ACH) | >12 | ≥12 (for renovation or new construction) |
| Filtration efficiency | Supply: 99.97% @ 0.3 μm DOP (dioctylphthalate particles of 0.3 μm diameter) Return: none required (If the patient requires both PE and AII, return air should be HEPA-filtered or otherwise exhausted to the outside) |
Supply: 90% (dust spot test) Return: 99.97% @ 0.3 μm DOP (dioctylphthalate particles of 0.3 μm diameter); HEPA filtration of exhaust air from AII rooms should not be required, providing that the exhaust is properly located to prevent re-entry into the building. |
| Room airflow direction | Out to the adjacent area | In to the room |
| Clean-to-dirty airflow in room | Away from the patient (high-risk patient, immunosuppressed patient) | Towards the patient (airborne disease patient) |
| Ideal pressure differential | > + 8 Pa | > −2.5 Pa |
* Material in this table was compiled from references 35 and 120. Table adapted from and used with permission of the publisher of reference 35 (Lippincott Williams and Wilkins).
§ Pa is the abbreviation for Pascal, a metric unit of measurement for pressure based on air velocity; 250 Pa equals 1.0 inch water gauge.
Health-care professionals (e.g., infection control, hospital epidemiologists) must perform a risk assessment to determine the appropriate number of AII rooms (negative pressure) and/or PE rooms (positive pressure) to serve the patient population. The AIA guidelines require a certain number of AII rooms as a minimum, and it is important to refer to the edition under which the building was built for appropriate guidance.120
In large health-care facilities with central HVAC systems, sealed windows help to ensure the efficient operation of the system, especially with respect to creating and maintaining pressure differentials. Sealing the windows in PE areas helps minimize the risk of airborne contamination from the outside. One outbreak of aspergillosis among immunosuppressed patients in a hospital was attributed in part to an open window in the unit during a time when both construction and a fire happened nearby; sealing the window prevented further entry of fungal spores into the unit from the outside air.111 Additionally, all emergency exits (e.g., fire escapes and emergency doors) in PE wards should be kept closed (except during emergencies) and equipped with alarms.
e. Infection Control Impact of HVAC System Maintenance and Repair
A failure or malfunction of any component of the HVAC system may subject patients and staff to discomfort and exposure to airborne contaminants. Only limited information is available from formal studies on the infection-control implications of a complete air-handling system failure or shutdown for maintenance. Most experience has been derived from infectious disease outbreaks and adverse outcomes among high-risk patients when HVAC systems are poorly maintained. (See Table 7 for potential ventilation hazards, consequences, and correction measures.)
AIA guidelines prohibit U.S. hospitals and surgical centers from shutting down their HVAC systems for purposes other than required maintenance, filter changes, and construction.120 Airflow can be reduced; however, sufficient supply, return, and exhaust must be provided to maintain required pressure relationships when the space is not occupied. Maintaining these relationships can be accomplished with special drives on the air-handling units (i.e., a variable air ventilation [VAV] system).
Microorganisms proliferate in environments wherever air, dust, and water are present, and air-handling systems can be ideal environments for microbial growth.35 Properly engineered HVAC systems require routine maintenance and monitoring to provide acceptable indoor air quality efficiently and to minimize conditions that favor the proliferation of health-care associated pathogens.35, 249 Performance monitoring of the system includes determining pressure differentials across filters, regular inspection of system filters, DOP testing of HEPA filters, testing of low- or medium efficiency filters, and manometer tests for positive- and negative-pressure areas in accordance with nationally recognized standards, guidelines, and manufacturers’ recommendations. The use of hand-held, calibrated equipment that can provide a numerical reading on a daily basis is preferred for engineering purposes (A.Streifel, University of Minnesota, 2000).256 Several methods that provide a visual, qualitative measure of pressure differentials (i.e., airflow direction) include smoke-tube tests or placing flutter strips, ping-pong balls, or tissue in the air stream.
Preventive filter and duct maintenance (e.g., cleaning ductwork vents, replacing filters as needed, and properly disposing spent filters into plastic bags immediately upon removal) is important to prevent potential exposures of patients and staff during HVAC system shut-down. The frequency of filter inspection and the parameters of this inspection are established by each facility to meet their unique needs. Ductwork in older health-care facilities may have insulation on the interior surfaces that can trap contaminants. This insulation material tends to break down over time to be discharged from the HVAC system. Additionally, a malfunction of the air-intake system can overburden the filtering system and permit aerosolization of fungal pathogens. Keeping the intakes free from bird droppings, especially those from pigeons, helps to minimize the concentration of fungal spores entering from the outside.98
Accumulation of dust and moisture within HVAC systems increases the risk for spread of health-care– associated environmental fungi and bacteria. Clusters of infections caused by Aspergillus spp., P. aeruginosa, S. aureus, and Acinetobacter spp. have been linked to poorly maintained and/or malfunctioning air conditioning systems.68, 161, 257, 258 Efforts to limit excess humidity and moisture in the infrastructure and on air-stream surfaces in the HVAC system can minimize the proliferation and dispersion of fungal spores and waterborne bacteria throughout indoor air.259–262 Within the HVAC system, water is present in water-wash units, humidifying boxes, or cooling units. The dual-duct system may also create conditions of high humidity and excess moisture that favor fungal growth in drain pans as well as in fibrous insulation material that becomes damp as a result of the humid air passing over the hot stream and condensing.
If moisture is present in the HVAC system, periods of stagnation should be avoided. Bursts of organisms can be released upon system start-up, increasing the risk of airborne infection.206 Proper engineering of the HVAC system is critical to preventing dispersal of airborne organisms. In one hospital, endophthalmitis caused by Acremonium kiliense infection following cataract extraction in an ambulatory surgical center was traced to aerosols derived from the humidifier water in the ventilation system.206 The organism proliferated because the ventilation system was turned off routinely when the center was not in operation; the air was filtered before humidification, but not afterwards.
Most health-care facilities have contingency plans in case of disruption of HVAC services. These plans include back-up power generators that maintain the ventilation system in high-risk areas (e.g., operating rooms, intensive-care units, negative- and positive-pressure rooms, transplantation units, and oncology units). Alternative generators are required to engage within 10 seconds of a loss of main power. If the ventilation system is out of service, rendering indoor air stagnant, sufficient time must be allowed to clean the air and re-establish the appropriate number of ACH once the HVAC system begins to function again. Air filters may also need to be changed, because reactivation of the system can dislodge substantial amounts of dust and create a transient burst of fungal spores.
Duct cleaning in health-care facilities has benefits in terms of system performance, but its usefulness for infection control has not been conclusively determined. Duct cleaning typically involves using specialized tools to dislodge dirt and a high-powered vacuum cleaner to clean out debris.263 Some duct-cleaning services also apply chemical biocides or sealants to the inside surfaces of ducts to minimize fungal growth and prevent the release of particulate matter. The U.S. Environmental Protection Agency (EPA), however, has concerns with the use of sanitizers and/or disinfectants to treat the surfaces of ductwork, because the label indications for most of these products may not specifically include the use of the product in HVAC systems.264 Further, EPA has not evaluated the potency of disinfectants in such applications, nor has the agency examined the potential attendant health and safety risks. The EPA recommends that companies use only those chemical biocides that are registered for use in HVAC systems.264 Although infrequent cleaning of the exhaust ducts in AII areas has been documented as a cause of diminishing negative pressure and a decrease in the air exchange rates,214 no data indicate that duct cleaning, beyond what is recommended for optimal performance, improves indoor air quality or reduces the risk of infection. Exhaust return systems should be cleaned as part of routine system maintenance. Duct cleaning has not been shown to prevent any health problems,265 and EPA studies indicate that airborne particulate levels do not increase as a result of dirty air ducts, nor do they diminish after cleaning, presumably because much of the dirt inside air ducts adheres to duct surfaces and does not enter the conditioned space.265 Additional research is needed to determine if air-duct contamination can significantly increase the airborne infection risk in general areas of health-care facilities.
4. Construction, Renovation, Remediation, Repair, and Demolition
a. General Information
Environmental disturbances caused by construction and/or renovation and repair activities (e.g., disruption of the above-ceiling area, running cables through the ceiling, and structural repairs) in and near health-care facilities markedly increase the airborne Aspergillus spp. spore counts in the indoor air of such facilities, thereby increasing the risk for health-care associated aspergillosis among high-risk patients. Although one case of health-care associated aspergillosis is often difficult to link to a specific environmental exposure, the occurrence of temporarily clustered cases increase the likelihood that an environmental source within the facility may be identified and corrected.
Table 7. Ventilation hazards in health-care facilities that may be associated with increased potential of airborne disease transmission*
| Problem§ | Consequences | Possible solutions |
|---|---|---|
| Water-damaged building materials (18, 266) | Water leaks can soak wood, wall board, insulation, wall coverings, ceiling tiles, and carpeting. All of these materials can provide microbial habitat when wet. This is especially true for fungi growing on gypsum board. |
|
| Filter bypasses (17) | Rigorous air filtration requires air flow resistance. Air stream will elude filtration if openings are present because of filter damage or poor fit. |
|
| Improper fan setting (267) | Air must be delivered at design volume to maintain pressure balances. Air flow in special vent rooms reverses. |
|
| Ductwork disconnections (268) | Dislodged or leaky supply duct runs can spill into and leaky returns may draw from hidden areas. Pressure balance will be interrupted, and infectious material may be disturbed and entrained into hospital air supply. |
|
| Air flow impedance (213) | Debris, structural failure, or improperly adjusted dampers can block duct work and prevent designed air flow. |
|
| Open windows (96, 247) | Open windows can alter fan-induced pressure balance and allow dirty-to clean air flow. |
|
| Dirty window air conditioners (96, 269) | Dirt, moisture, and bird droppings can contaminate window air conditioners, which can then introduce infectious material into hospital rooms. |
|
| Inadequate filtration (270) | Infectious particles may pass through filters into vulnerable patient areas. |
|
| Maintenance disruptions (271) | Fan shut-offs, dislodged filter cake material contaminates downstream air supply and drain pans. This may compromise air flow in special ventilation areas. |
|
| Excessive moisture in the HVAC system (120) | Chronically damp internal lining of the HVAC system, excessive condensate, and drip pans with stagnant water may result from this problem. |
|
| Duct contamination (18, 272) | Debris is released during maintenance or cleaning. |
|
* Reprinted with permission of the publisher of reference 35 (Lippincott Williams and Wilkins).
§ Numbers in parentheses are reference citations.
Construction, renovation, repair, and demolition activities in health-care facilities require substantial planning and coordination to minimize the risk for airborne infection both during projects and after their completion. Several organizations and experts have endorsed a multi-disciplinary team approach (Box 4) to coordinate the various stages of construction activities (e.g., project inception, project implementation, final walk-through, and completion).120, 249, 250, 273–276 Environmental services, employee health, engineering, and infection control must be represented in construction planning and design meetings should be convened with architects and design engineers. The number of members and disciplines represented is a function of the complexity of a project. Smaller, less complex projects and maintenance may require a minimal number of members beyond the core representation from engineering, infection control, environmental services, and the directors of the
specialized departments.
Box 4. Suggested members and functions of a multi-disciplinary coordination team for construction, renovation, repair, and demolition projects
Members
- Infection-control personnel, including hospital epidemiologists
- Laboratory personnel
- Facility administrators or their designated representatives, facility managers
- Director of engineering
- Risk-management personnel
- Directors of specialized programs (e.g., transplantation, oncology and ICU [intensive care unit] programs)
- Employee safety personnel, industrial hygienists, and regulatory affairs personnel
- Environmental services personnel Information systems personnel
- Construction administrators or their designated representatives
- Architects, design engineers, project managers, and contractors
Functions and responsibilities
- Coordinate members’ input in developing a comprehensive project management plan.
- Conduct a risk assessment of the project to determine potential hazards to susceptible patients.
- Prevent unnecessary exposures of patients, visitors, and staff to infectious agents.
- Oversee all infection-control aspects of construction activities.
- Establish site-specific infection-control protocols for specialized areas.
- Provide education about the infection-control impact of construction to staff and construction workers.
- Ensure compliance with technical standards, contract provisions, and regulations.
- Establish a mechanism to address and correct problems quickly.
- Develop contingency plans for emergency response to power failures, water supply disruptions, and fires.
- Provide a water-damage management plan (including drying protocols) for handling water intrusion from floods, leaks, and condensation.
- Develop a plan for structural maintenance.
Education of maintenance and construction workers, health-care staff caring for high-risk patients, and persons responsible for controlling indoor air quality heightens awareness that minimizing dust and moisture intrusion from construction sites into high-risk patient-care areas helps to maintain a safe environment.120, 250, 271, 275–278 Visual and printed educational materials should be provided in the language spoken by the workers. Staff and construction workers also need to be aware of the potentially catastrophic consequences of dust and moisture intrusion when an HVAC system or water system fails during construction or repair; action plans to deal quickly with these emergencies should be developed in advance and kept on file. Incorporation of specific standards into construction contracts may help to prevent departures from recommended practices as projects progress. Establishing specific lines of communication is important to address problems (e.g., dust control, indoor air quality, noise levels, and vibrations), resolve complaints, and keep projects moving toward completion. Health-care facility staff should develop a mechanism to monitor worker adherence to infection-control guidelines on a daily basis in and around the construction site for the duration of the project.
b. Preliminary Considerations
The three major topics to consider before initiating any construction or repair activity are as follows:
- design and function of the new structure or area,
- assessment of environmental risks for airborne disease and opportunities for prevention, and
- measures to contain dust and moisture during construction or repairs.
A checklist of design and function considerations can help to ensure that a planned structure or area can be easily serviced and maintained for environmental infection control (Box 5) .17, 250, 273, 275–277 Specifications for the construction, renovation, remodeling, and maintenance of health-care facilities are outlined in the AIA document, Guidelines for Design and Construction of Hospitals and Health Care Facilities. 120, 275
Box 5. Construction design and function considerations for environmental infection control
- Location of sinks and dispensers for handwashing products and hand hygiene products
- Types of faucets (e.g., aerated vs. non-aerated)
- Air-handling systems engineered for optimal performance, easy maintenance, and repair
- ACH and pressure differentials to accommodate special patient-care areas
- Location of fixed sharps containers
- Types of surface finishes (e.g., porous vs. non-porous)
- Well-caulked walls with minimal seams
- Location of adequate storage and supply areas
- Appropriate location of medicine preparations areas (e.g., >3 ft. from a sink)
- Appropriate location and type of ice machines (e.g., preferably ice dispensers rather than ice bins)
- Appropriate materials for sinks and wall coverings
- Appropriate traffic flow (e.g., no “dirty” movement through “clean” areas)
- Isolation rooms with anterooms as appropriate
- Appropriate flooring (e.g., seamless floors in dialysis units)
- Sensible use carpeting (e.g., avoiding use of carpeting in special care areas or areas likely to become wet)*
- Convenient location of soiled utility areas
- Properly engineered areas for linen services and solid waste management
- Location of main generator to minimize the risk of system failure from flooding or other emergency
- Installation guidelines for sheetrock
* Use of carpet cleaning methods (e.g., “bonneting”) that disperse microorganisms into the air may increase the risk of airborne infection among at-risk patients, especially if they are in the vicinity of the cleaning activity.111
Proactive strategies can help prevent environmentally mediated airborne infections in health-care facilities during demolition, construction, and renovation. The potential presence of dust and moisture and their contribution to health-care associated infections must be critically evaluated early in the planning of any demolition, construction, renovation, and repairs.120, 250, 251, 273, 274, 276–279 Consideration must extend beyond dust generated by major projects to include dust that can become airborne if disturbed during routine maintenance and minor renovation activities (e.g., exposure of ceiling spaces for inspection; installation of conduits, cable, or sprinkler systems; rewiring; and structural repairs or replacement).273, 276, 277 Other projects that can compromise indoor air quality include construction and repair jobs that inadvertently allow substantial amounts of raw, unfiltered outdoor air to enter the facility (e.g., repair of elevators and elevator shafts) and activities that dampen any structure, area, or item made of porous materials or characterized by cracks and crevices (e.g., sink cabinets in need of repair, carpets, ceilings, floors, walls, vinyl wall coverings, upholstery, drapes, and countertops).18, 273, 277 Molds grow and proliferate on these surfaces when they become and remain wet.21, 120, 250, 266, 270, 272, 280 Scrubbable materials are preferred for use in patient-care areas.
Containment measures for dust and/or moisture control are dictated by the location of the construction site. Outdoor demolition and construction require actions to keep dust and moisture out of the facility (e.g., sealing windows and vents and keeping doors closed or sealed). Containment of dust and moisture generated from construction inside a facility requires barrier structures (either pre-fabricated or constructed of more durable materials as needed) and engineering controls to clean the air in and around the construction or repair site.
c. Infection-Control Risk Assessment
An infection-control risk assessment (ICRA) conducted before initiating repairs, demolition, construction, or renovation activities can identify potential exposures of susceptible patients to dust and moisture and determine the need for dust and moisture containment measures. This assessment centers on the type and extent of the construction or repairs in the work area but may also need to include adjacent patient-care areas, supply storage, and areas on levels above and below the proposed project. An example of designing an ICRA as a matrix, the policy for performing an ICRA and implementing its results, and a sample permit form that streamlines the communication process are available.281 Knowledge of the air flow patterns and pressure differentials helps minimize or eliminate the inadvertent dispersion of dust that could contaminate air space, patient-care items, and surfaces.57, 282, 283 A recent aspergillosis outbreak among oncology patients was attributed to depressurization of the building housing the HSCT unit while construction was underway in an adjacent building. Pressure readings in the affected building (including 12 of 25 HSCT-patient rooms) ranged from 0.1 Pa–5.8 Pa. Unfiltered outdoor air flowed into the building through doors and windows, exposing patients in the HSCT unit to fungal spores.283 During long-term projects, providing temporary essential services (e.g., toilet facilities) and conveniences (e.g., vending machines) to construction workers within the site will help to minimize traffic in and out of the area. The type of barrier systems necessary for the scope of the project must be defined.12, 120, 250, 279, 284
Depending on the location and extent of the construction, patients may need to be relocated to other areas in the facility not affected by construction dust.51, 285 Such relocation might be especially prudent when construction takes place within units housing immunocompromised patients (e.g., severely neutropenic patients and patients on corticosteroid therapy). Advance assessment of high-risk locations and planning for the possible transport of patients to other departments can minimize delays and waiting time in hallways.51 Although hospitals have provided immunocompromised patients with some form of respiratory protection for use outside their rooms, the issue is complex and remains unresolved until more research can be done. Previous guidance on this issue has been inconsistent.9 Protective respirators (i.e., N95) were well tolerated by patients when used to prevent further cases of construction-related aspergillosis in a recent outbreak.283 The routine use of the N95 respirator by patients, however, has not been evaluated for preventing exposure to fungal spores during periods of non-construction. Although health-care workers who would be using the N95 respirator for personal respiratory protect must be fit-tested, there is no indication that either patients or visitors should undergo fit-testing.
Surveillance activities should augment preventive strategies during construction projects.3, 4, 20, 110, 286, 287 By determining baseline levels of health-care acquired airborne and waterborne infections, infection-control staff can monitor changes in infection rates and patterns during and immediately after construction, renovations, or repairs.3
d. Air Sampling
Air sampling in health-care facilities may be conducted both during periods of construction and on a periodic basis to determine indoor air quality, efficacy of dust-control measures, or air-handling system performance via parametric monitoring. Parametric monitoring consists of measuring the physical periodic assessment of the system (e.g., air flow direction and pressure, ACH, and filter efficiency) can give assurance of proper ventilation, especially for special care areas and operating rooms.288
Air sampling is used to detect aerosols (i.e., particles or microorganisms). Particulate sampling (i.e., total numbers and size range of particulates) is a practical method for evaluating the infection-control performance of the HVAC system, with an emphasis on filter efficiency in removing respirable particles (<5 μm in diameter) or larger particles from the air. Particle size is reported in terms of the mass median aerodynamic diameter (MMAD), whereas count median aerodynamic diameter (CMAD) is useful with respect to particle concentrations.
Particle counts in a given air space within the health-care facility should be evaluated against counts obtained in a comparison area. Particle counts indoors are commonly compared with the particulate levels of the outdoor air. This approach determines the “rank order” air quality from “dirty” (i.e., the outdoor air) to “clean” (i.e., air filtered through high-efficiency filters [90%–95% filtration]) to “cleanest” (i.e., HEPA-filtered air).288 Comparisons from one indoor area to another may also provide useful information about the magnitude of an indoor air-quality problem. Making rank-order comparisons between clean, highly-filtered areas and dirty areas and/or outdoors is one way to interpret sampling results in the absence of air quality and action level standards.35, 289
In addition to verifying filter performance, particle counts can help determine if barriers and efforts to control dust dispersion from construction are effective. This type of monitoring is helpful when performed at various times and barrier perimeter locations during the project. Gaps or breaks in the barriers’ joints or seals can then be identified and repaired. The American Conference of Governmental Industrial Hygienists (ACGIH) has set a threshold limit value-time weighted average (TLV®-TWA) of 10 mg/m3 for nuisance dust that contains no asbestos and <1% crystalline silica.290 Alternatively, OSHA has set permissible exposure limits (PELs) for inert or nuisance dust as follows: respirable fraction at 5 mg/m3 and total dust at 15 mg/m3 . 291 Although these standards are not measures of a bioaerosol, they are used for indoor air quality assessment in occupational settings and may be useful criteria in construction areas. Application of ACGIH guidance to health-care settings has not been standardized, but particulate counts in health-care facilities are likely to be well below this threshold value and approaching clean-room standards in certain care areas (e.g., operating rooms).100
Particle counters and anemometers are used in particulate evaluation. The anemometer measures air flow velocity, which can be used to determine sample volumes. Particulate sampling usually does not require microbiology laboratory services for the reporting of results.
Microbiologic sampling of air in health-care facilities remains controversial because of currently unresolved technical limitations and the need for substantial laboratory support (Box 6). Infection-control professionals, laboratorians, and engineers should determine if microbiologic and/or particle sampling is warranted and assess proposed methods for sampling. The most significant technical limitation of air sampling for airborne fungal agents is the lack of standards linking fungal spore levels with infection rates. Despite this limitation, several health-care institutions have opted to use microbiologic sampling when construction projects are anticipated and/or underway in efforts to assess the safety of the environment for immunocompromised patients.35, 289 Microbiologic air sampling should be limited to assays for airborne fungi; of those, the thermotolerant fungi (i.e., those capable of growing at 95°F–98.6°F [35°C–37°C]) are of particular concern because of their pathogenicity in immunocompromised hosts.35 Use of selective media (e.g., Sabouraud dextrose agar and inhibitory mold agar) helps with the initial identification of recovered organisms.
Microbiologic sampling for fungal spores performed as part of various airborne disease outbreak investigations has also been problematic.18, 49, 106, 111, 112, 289 The precise source of a fungus is often difficult to trace with certainty, and sampling conducted after exposure may neither reflect the circumstances that were linked to infection nor distinguish between health-care acquired and community-acquired infections. Because fungal strains may fluctuate rapidly in the environment, health-care acquired Aspergillus spp. infection cannot be confirmed or excluded if the infecting strain is not found in the health-care setting.287 Sensitive molecular typing methods (e.g., randomly amplified polymorphic DNA (RAPD) techniques and a more recent DNA fingerprinting technique that detects restriction fragment length polymorphisms in fungal genomic DNA) to identify strain differences among Aspergillus spp., however, are becoming increasingly used in epidemiologic investigations of health-care acquired fungal infection (A.Streifel, University of Minnesota, 2000).68, 110, 286, 287, 292–296 During case cluster evaluation, microbiologic sampling may provide an isolate from the environment for molecular typing and comparison with patient isolates. Therefore, it may be prudent for the clinical laboratory to save Aspergillus spp. isolated from colonizations and invasive disease cases among patients in PE, oncology, and transplant services for these purposes.
Box 6. Unresolved issues associated with microbiologic air sampling*
- Lack of standards linking fungal spore levels with infection rates (i.e., no safe level of exposure)
- Lack of standard protocols for testing (e.g., sampling intervals, number of samples, sampling locations)
- Need for substantial laboratory support
- Culture issues (e.g., false negatives, insensitivity, lag time between sampling and recording the results)
- New, complex polymerase chain reaction (PCR) analytical methods
- Unknown incubation period for Aspergillus spp. infection
- Variability of sampler readings
- Sensitivity of the sampler used (i.e., the volumes of air sampled)
- Lack of details in the literature about describing sampling circumstances (e.g., unoccupied rooms vs. ongoing activities in rooms, expected fungal concentrations, and rate of outdoor air penetration)
- Lack of correlation between fungal species and strains from the environment and clinical specimens
- Confounding variables with high-risk patients (e.g., visitors and time spent outside of protective environment [PE] without respiratory protection)
- Need for determination of ideal temperature for incubating fungal cultures (95°F [35°C] is the most commonly used temperature)
* Material in this box is compiled from references 35, 100, 222, 289, 297.
Sedimentation methods using settle plates and volumetric sampling methods using solid impactors are commonly employed when sampling air for bacteria and fungi. Settle plates have been used by numerous investigators to detect airborne bacteria or to measure air quality during medical procedures (e.g., surgery).17, 60, 97, 151, 161, 287 Settle plates, because they rely on gravity during sampling, tend to select for larger particles and lack sensitivity for respirable particles (e.g., individual fungal spores), especially in highly-filtered environments. Therefore, they are considered impractical for general use.35, 289, 298–301 Settle plates, however, may detect fungi aerosolized during medical procedures (e.g., during wound dressing changes), as described in a recent outbreak of aspergillosis among liver transplant patients.302
The use of slit or sieve impactor samplers capable of collecting large volumes of air in short periods of time are needed to detect low numbers of fungal spores in highly filtered areas.35, 289 In some outbreaks, aspergillosis cases have occurred when fungal spore concentrations in PE ambient air ranged as low as 0.9–2.2 colony-forming units per cubic meter (CFU/m3 ) of air.18, 94 On the basis of the expected spore counts in the ambient air and the performance parameters of various types of volumetric air samplers, investigators of a recent aspergillosis outbreak have suggested that an air volume of at least 1000 L (1 m3 ) should be considered when sampling highly filtered areas.283 Investigators have also suggested limits of 15 CFU/m3 for gross colony counts of fungal organisms and <0.1 CFU/m3 for Aspergillus fumigatus and other potentially opportunistic fungi in heavily filtered areas (≥12 ACH and filtration of ≥99.97% efficiency).120 No correlation of these values with the incidence of health-care– associated fungal infection rates has been reported.
Air sampling in health-care facilities, whether used to monitor air quality during construction, to verify filter efficiency, or to commission new space prior to occupancy, requires careful notation of the circumstances of sampling. Most air sampling is performed under undisturbed conditions. However, when the air is sampled during or after human activity (e.g., walking and vacuuming), a higher number of airborne microorganisms likely is detected.297 The contribution of human activity to the significance of air sampling and its impact on health-care associated infection rates remain to be defined. Comparing microbiologic sampling results from a target area (e.g., an area of construction) to those from an unaffected location in the facility can provide information about distribution and concentration of potential airborne pathogens. A comparison of microbial species densities in outdoor air versus indoor air has been used to help pinpoint fungal spore bursts. Fungal spore densities in outdoor air are variable, although the degree of variation with the seasons appears to be more dramatic in the United States than in Europe.92, 287, 303
Particulate and microbiologic air sampling have been used when commissioning new HVAC system installations; however, such sampling is particularly important for newly constructed or renovated PE or operating rooms. Particulate sampling is used as part of a battery of tests to determine if a new HVAC system is performing to specifications for filtration and the proper number of ACH.268, 288, 304 Microbiologic air sampling, however, remains controversial in this application, because no standards for comparison purposes have been determined. If performed, sampling should be limited to determining the density of fungal spores per unit volume of air space. High numbers of spores may indicate contamination of air-handling system components prior to installation or a system deficiency when culture results are compared with known filter efficiencies and rates of air exchange.
e. External Demolition and Construction
External demolition, planned building implosions, and dirt excavation generate considerable dust and debris that can contain airborne microorganisms. In one study, peak concentrations in outdoor air at grade level and HVAC intakes during site excavation averaged 20,000 CFU/m3 for all fungi and 500 CFU/m3 for Aspergillus fumigatus, compared with 19 CFU/m3 and 4 CFU/m3 , respectively, in the absence of construction.280 Many health-care institutions are located in large, urban areas; building implosions are becoming a more frequent concern. Infection-control risk assessment teams, particularly those in facilities located in urban renewal areas, would benefit by developing risk management strategies for external demolition and construction as a standing policy. In light of the events of 11 September 2001, it may be necessary for the team to identify those dust exclusion measures that can be implemented rapidly in response to emergency situations (Table 8). Issues to be reviewed prior to demolition include
- proximity of the air intake system to the work site,
- adequacy of window seals and door seals,
- proximity of areas frequented by immunocompromised patients, and
- location of the underground utilities (D. Erickson, ASHE, 2000).120, 250, 273, 276, 277, 280, 305
Table 8. Strategies to reduce dust and moisture intrusion during external demolition and construction
| Item | Recommendation |
|---|---|
| Demolition site |
|
| Dust-generating equipment |
|
| Construction materials storage |
|
| Adjacent air intakes |
|
| HVAC system |
|
| Filters |
|
| Windows |
|
| Doors |
|
| Water utilities |
|
| Medical gas piping |
|
| Rooftops |
|
| Dust generation |
|
| Immunocompromised patients |
|
| Pedestrian traffic |
|
| Truck traffic |
|
| Education and awareness+ |
|
+ When health-care facilities have immunosuppressed patients in their census, telephoning the city building department each month to find out if buildings are scheduled for demolition is prudent.
Minimizing the entry of outside dust into the HVAC system is crucial in reducing the risk for airborne contaminants. Facility engineers should be consulted about the potential impact of shutting down the system or increasing the filtration. Selected air handlers, especially those located close to excavation sites, may have to be shut off temporarily to keep from overloading the system with dust and debris. Care is needed to avoid significant facility-wide reductions in pressure differentials that may cause the building to become negatively pressured relative to the outside. To prevent excessive particulate overload and subsequent reductions in effectiveness of intake air systems that cannot be shut off temporarily, air filters must be inspected frequently for proper installation and function. Excessive dust penetration can be avoided if recirculated air is maximally utilized while outdoor air intakes are shut down. Scheduling demolition and excavation during the winter, when Aspergillus spp. spores may be present in lower numbers, can help, although seasonal variations in spore density differ around the world.92, 287, 303
Dust control can be managed by misting the dirt and debris during heavy dust-generating activities. To decrease the amount of aerosols from excavation and demolition projects, nearby windows, especially in areas housing immunocompromised patients, can be sealed and window and door frames caulked or weather-stripped to prevent dust intrusion.50, 301, 306 Monitoring for adherence to these control measures throughout demolition or excavation is crucial. Diverting pedestrian traffic away from the construction sites decreases the amount of dust tracked back into the health-care facility and minimizes exposure of high-risk patients to environmental pathogens. Additionally, closing entrances near construction or demolition sites might be beneficial; if this is not practical, creating an air lock (i.e., pressurizing the entry way) is another option.
f. Internal Demolition, Construction, Renovations, and Repairs
The focus of a properly implemented infection-control program during interior construction and repairs is containment of dust and moisture. This objective is achieved by
- educating construction workers about the importance of control measures
- preparing the site;
- notifying and issuing advisories for staff, patients, and visitors;
- moving staff and patients and relocating patients as needed;
- issuing standards of practice and precautions during activities and maintenance;
- monitoring for adherence to control measures during construction and providing prompt feedback about lapses in control
- monitoring HVAC performance;
- implementing daily clean-up, terminal cleaning and removal of debris upon completion; and
- ensuring the integrity of the water system during and after construction.
These activities should be coordinated with engineering staff and infection-control professionals.
Physical barriers capable of containing smoke and dust will confine dispersed fungal spores to the construction zone.279, 284, 307, 308 The specific type of physical barrier required depends on the project’s scope and duration and on local fire codes. Short-term projects that result in minimal dust dispersion (e.g., installation of new cables or wiring above ceiling tiles) require only portable plastic enclosures with negative pressure and HEPA filtration of the exhaust air from the enclosed work area. The placement of a portable industrial-grade HEPA filter device capable of filtration rate of 300–800 ft3 /min. adjacent to the work area will help to remove fungal spores, but its efficacy is dependent on the supplied ACH and size of the area. If the project is extensive but short-term, dust-abatement, fire-resistant plastic curtains (e.g., Visqueen®) may be adequate. These should be completely airtight and sealed from ceiling to floor with overlapping curtains;276, 277, 309 holes, tears, or other perforations should be repaired promptly with tape. A portable, industrial-grade HEPA filter unit on continuous operation is needed within the contained area, with the filtered air exhausted to the outside of the work zone. Patients should not remain in the room when dust-generating activities are performed. Tools to assist the decision-making process regarding selection of barriers based on an ICRA approach are available.281
More elaborate barriers are indicated for long-term projects that generate moderate to large amounts of dust. These barrier structures typically consist of rigid, noncombustible walls constructed from sheet rock, drywall, plywood, or plaster board and covered with sheet plastic (e.g., Visqueen®). Barrier requirements to prevent the intrusion of dust into patient-care areas include
- installing a plastic dust abatement curtain before construction of the rigid barrier
- sealing and taping all joint edges including the top and bottom;
- extending the barrier from floor to floor, which takes into account the space [approximately 2–8 ft.] above the finished, lay-down ceiling; and
- fitting or sealing any temporary doors connecting the construction zone to the adjacent area. (See Box 7 for a list of the various construction and repair activities that require the use of some type of barrier.)
Box 7. Construction/repair projects that require barrier structures*
- Demolition of walls, wallboard, plaster, ceramic tiles, ceiling tiles, and ceilings
- Removal of flooring and carpeting, windows and doors, and casework
- Working with sinks and plumbing that could result in aerosolization of water in high-risk areas
- Exposure of ceiling spaces for demolition and for installation or rerouting of utility services (e.g., rewiring, electrical conduction installation, HVAC ductwork, and piping)
- Crawling into ceiling spaces for inspection in a manner that may dislodge dust
- Demolition, repair, or construction of elevator shafts
- Repairing water damage
* Material for this box was compiled from references 120, 250, 273, 276, 277.
Dust and moisture abatement and control rely primarily on the impermeable barrier containment approach; as construction continues, numerous opportunities can lead to dispersion of dust to other areas of the health-care facility. Infection-control measures that augment the use of barrier containment should be undertaken (Table 9).
Dust-control measures for clinical laboratories are an essential part of the infection-control strategy during hospital construction or renovation. Use of plastic or solid barriers may be needed if the ICRA determines that air flow from construction areas may introduce airborne contaminants into the laboratory space. In one facility, pseudofungemia clusters attributed to Aspergillus spp. and Penicillium spp. were linked to improper air flow patterns and construction projects adjacent to the laboratory; intrusion of dust and spores into a biological safety cabinet from construction activity immediately next to the cabinet resulted in a cluster of cultures contaminated with Aspergillus niger. 310, 311 Reportedly, no barrier containment was used and the HEPA filtration system was overloaded with dust. In addition, an outbreak of pseudobacteremia caused by Bacillus spp. occurred in another hospital during construction above a storage area for blood culture bottles.207 Airborne spread of Bacillus spp. spores resulted in contamination of the bottles’ plastic lids, which were not disinfected or handled with proper aseptic technique prior to collection of blood samples.
Table 9. Infection-control measures for internal construction and repair projects*+
| Infection-control measure | Steps for implementation |
|---|---|
| Prepare for the project. |
|
| Educate staff and construction workers. |
|
| Issue hazard and warning notices. |
|
| Relocate high-risk patients as needed, especially if the construction is in or adjacent to a PE area. |
|
| Establish alternative traffic patterns for staff, patients, visitors, and construction workers. |
|
| Erect appropriate barrier containment. |
|
| Establish proper ventilation. |
|
| Control solid debris. |
|
| Control water damage. |
|
| Control dust in air and on surfaces. |
|
| Complete the project. |
|
* Material in this table includes information from D. Erickson, ASHE, 2000.
+ Material in this table was compiled from references 19, 51, 67, 80, 106, 120, 250, 266, 273, 276–278, 280, 285, 309, 312–315.
5. Environmental Infection-Control Measures for Special Health-Care Settings
Areas in health-care facilities that require special ventilation include
- operating rooms
- PE rooms used by high-risk, immunocompromised patients; and
- AII rooms for isolation of patients with airborne infections (e.g., those caused by M. tuberculosis, VZV, or measles virus).
The number of rooms required for PE and AII are determined by a risk assessment of the health-care facility.6 Continuous, visual monitoring of air flow direction is required for new or renovated pressurized rooms. 120, 256
a. Protective Environments (PE)
Although the exact configuration and specifications of PEs might differ among hospitals, these care areas for high-risk, immunocompromised patients are designed to minimize fungal spore counts in air by maintaining
- filtration of incoming air by using central or point-of-use HEPA filters
- directed room air flow [i.e., from supply on one side of the room, across the patient, and out through the exhaust on the opposite side of the room];
- positive room air pressure of 2.5 Pa [0.01″ water gauge] relative to the corridor;
- well-sealed rooms; and
- ≥12 ACH.44, 120, 251, 254, 316–319
Air flow rates must be adjusted accordingly to ensure sufficient ACH, and these rates vary depending on certain factors (e.g., room air leakage area). For example, to provide ≥12 ACH in a typical patient room with 0.5 sq. ft. air leakage, the air flow rate will be minimally 125 cubic feet/min (cfm).320, 321 Higher air flow rates may be needed. A general ventilation diagram for a positive-pressure room is given in Figure 2. Directed room air flow in PE rooms is not laminar; parallel air streams are not generated. Studies attempting to demonstrate patient benefit from laminar air flow in a PE setting are equivocal.316, 318, 319, 322 – 327
Air flow direction at the entrances to these areas should be maintained and verified, preferably on a daily basis, using either a visual means of indication (e.g., smoke tubes and flutter strips) or manometers. Permanent installation of a visual monitoring device is indicated for new PE construction and renovation.120 Facility service structures can interfere with the proper unidirectional air flow from the patients’ rooms to the adjacent corridor. In one outbreak investigation, Aspergillus spp. infections in a critical care unit may have been associated with a pneumatic specimen transport system, a textile disposal duct system, and central vacuum lines for housekeeping, all of which disrupted proper air flow from the patients’ rooms to the outside and allowed entry of fungal spores into the unit (M.McNeil, CDC, 2000).
Figure 2. Example of positive-pressure room control for protection from airborne environmental microbes (PE)* + § ¶
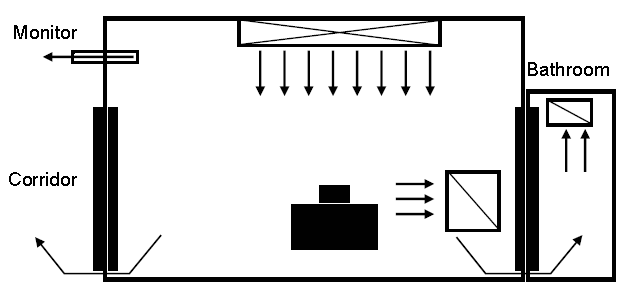
* Stacked black boxes represent patient’s bed. Long open box with cross-hatch represents supply air. Open boxes with single, diagonal slashes represent air exhaust registers. Arrows indicate directions of air flow.
+ Possible uses include immunocompromised patient rooms (e.g., hematopoietic stem cell transplant or solid organ transplant procedure rooms) and orthopedic operating rooms.
§ Positive-pressure room engineering features include positive pressure (greater supply than exhaust air volume); pressure differential range of 2.5–8 Pa (0.01–0.03-in. water gauge), ideal at 8 Pa; air flow volume differential >125-cfm supply versus exhaust; sealed room, approximately 0.5-sq. ft. leakage; clean to dirty air flow; monitoring; ≥12 air changes per hour (ACH); and return air if refiltered.
¶ This diagram is a generic illustration of air flow in a typical installation. Alternative air flow arrangements are recognized. Adapted and used with permission from A. Streifel and the publisher of reference 328 (Penton Media, Inc.)
The use of surface fungicide treatments is becoming more common, especially for building materials.329 Copper-based compounds have demonstrated anti-fungal activity and are often applied to wood or paint. Copper-8-quinolinolate was used on environmental surfaces contaminated with Aspergillus spp. to control one reported outbreak of aspergillosis.310 The compound was also incorporated into the fireproofing material of a newly constructed hospital to help decrease the environmental spore burden.316
b. Airborne Infection Isolation (AII)
Acute-care inpatient facilities need at least one room equipped to house patients with airborne infectious disease. Every health-care facility, including ambulatory and long-term care facilities, should undertake an ICRA to identify the need for AII areas. Once the need is established, the appropriate ventilation equipment can be identified. Air handling systems for this purpose need not be restricted to central systems. Guidelines for the prevention of health-care acquired TB have been published in response to multiple reports of health-care associated transmission of multi-drug resistant strains.4, 330 In reports documenting health-care acquired TB, investigators have noted a failure to comply fully with prevention measures in established guidelines.331 – 345 These gaps highlight the importance of prompt recognition of the disease, isolation of patients, proper treatment, and engineering controls. AII rooms are also appropriate for the care and management of smallpox patients.6 Environmental infection control with respect to smallpox is currently being revisited (see Appendix E).
Salient features of engineering controls for AII areas include
- use of negative pressure rooms with close monitoring of air flow direction using manometers or temporary or installed visual indicators [e.g., smoke tubes and flutter strips] placed in the room with the door closed
- minimum 6 ACH for existing facilities, ≥12 ACH for areas under renovation or for new construction; and
- air from negative pressure rooms and treatment rooms exhausted directly to the outside if possible.4, 120, 248
As with PE, airflow rates need to be determined to ensure the proper numbers of ACH.320, 321 AII rooms can be constructed either with (Figure 3) or without (Figure 4) an anteroom. When the recirculation of air from AII rooms is unavoidable, HEPA filters should be installed in the exhaust duct leading from the room to the general ventilation system. In addition to UVGI fixtures in the room, UVGI can be placed in the ducts as an adjunct measure to HEPA filtration, but it can not replace the HEPA filter.4, 346 A UVGI fixture placed in the upper room, coupled with a minimum of 6 ACH, also provides adequate air cleaning.248
Figure 3. Example of negative-pressure room control for airborne infection isolation (AII)* + §¶
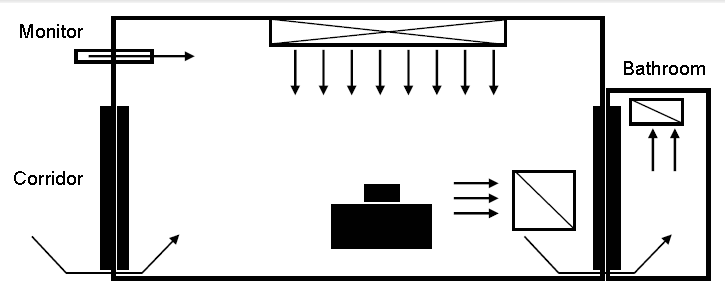
* Stacked black boxes represent patient’s bed. Long open box with cross-hatch represents supply air. Open boxes with single, diagonal slashes represent air exhaust registers. Arrows indicate direction of air flow.
+ Possible uses include treatment or procedure rooms, bronchoscopy rooms, and autopsy.
§ Negative-pressure room engineering features include negative pressure (greater exhaust than supply air volume); pressure differential of 2.5 Pa (0.01-in. water gauge); air flow volume differential >125-cfm exhaust versus supply; sealed room, approximately 0.5-sq. ft. leakage; clean to dirty air flow; monitoring; ≥12 air changes per hour (ACH) new or renovation, 6 ACH existing; and exhaust to outside or HEPA-filtered if recirculated.
¶ This diagram is a generic illustration of air flow in a typical installation. Alternative air flow arrangements are recognized. Adapted and used with permission from A. Streifel and the publisher of reference 328 (Penton Media, Inc.)
One of the components of airborne infection isolation is respiratory protection for health-care workers and visitors when entering AII rooms.4, 6, 347 Recommendations of the type of respiratory protection are dependent on the patient’s airborne infection (indicating the need for AII) and the risk of infection to persons entering the AII room. A more in-depth discussion of respiratory protection in this instance is presented in the current isolation guideline;6 a revision of this guideline is in development. Cough-inducing procedures (e.g., endotracheal intubation and suctioning of known or suspected TB patients, diagnostic sputum induction, aerosol treatments, and bronchoscopy) require similar precautions.348–350
Additional engineering measures are necessary for the management of patients requiring PE (i.e., allogeneic HSCT patients) who concurrently have airborne infection. For this type of patient treatment, an anteroom (Figure 4) is required in new construction and renovation as per AIA guidelines.120
Figure 4. Example of airborne infection isolation (AII) room with anteroom and neutral anteroom* + §
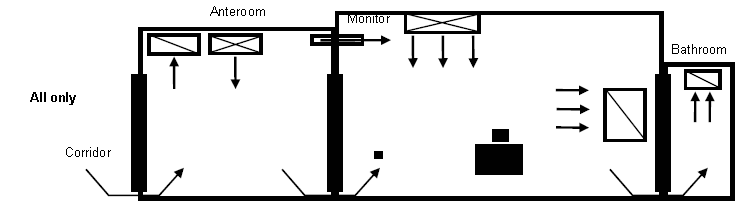
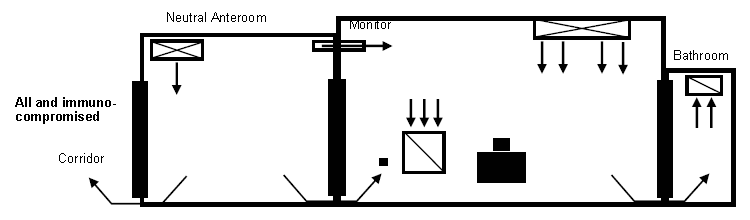
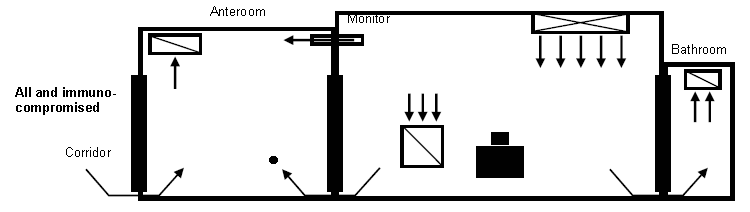
* The top diagram indicates air flow patterns when patient with only airborne infectious disease occupies room. Middle and bottom diagrams indicate recommended air flow patterns when room is occupied by immunocompromised patient with airborne infectious disease. Stacked black boxes represent patient beds. Long open boxes with cross-hatches represent supply air. Open boxes with single, diagonal slashes represent air exhaust registers. Arrows indicate directions of air flow.
+ AII isolation room with anteroom engineering features include
- pressure differential of 2.5 Pa (0.01-in. water gauge) measured at the door between patient room and anteroom;
- air flow volume differential >125-cfm. depending on anteroom air flow direction (pressurized versus depressurized);
- 38 sealed room with approximately 0.5-sq. ft. leakage;
- clean to dirty air flow
- monitoring;
- ≥12 air changes per hour (ACH) new or renovation, 6 ACH existing; and
- anteroom air flow patterns. The small ■ in panels 1 and 2 indicate the anteroom is pressurized (supply versus exhaust), while the small • in panel 3 indicates the anteroom is depressurized (exhaust versus supply).
§ Used with permission of A. Streifel, University of Minnesota
The pressure differential of an anteroom can be positive or negative relative to the patient in the room.120 An anteroom can act as an airlock (Figure 4). If the anteroom is positive relative to the air space in the patient’s room, staff members do not have to mask prior to entry into the anteroom if air is directly exhausted to the outside and a minimum of 10 ACH (Figure 4, top diagram).120 When an anteroom is negative relative to both the AII room and the corridor, health-care workers must mask prior to entering the anteroom (Figure 4, bottom diagram). If an AII room with an anteroom is not available, use of a portable, industrial-grade HEPA filter unit may help to increase the number of ACHs while facilitating the removal of fungal spores; however, a fresh air source must be present to achieve the proper air exchange rate. Incoming ambient air should receive HEPA filtration.
c. Operating Rooms
Operating room air may contain microorganisms, dust, aerosol, lint, skin squamous epithelial cells, and respiratory droplets. The microbial level in operating room air is directly proportional to the number of people moving in the room.351 One study documented lower infection rates with coagulase-negative staphylococci among patients when operating room traffic during the surgical procedure was limited.352 Therefore, efforts should be made to minimize personnel traffic during operations. Outbreaks of SSIs caused by group A beta-hemolytic streptococci have been traced to airborne transmission from colonized operating-room personnel to patients.150–154 Several potential health-care associated pathogens (e.g., Staphylococcus aureus and Staphylococcus epidermidis) and drug-resistant organisms have also been recovered from areas adjacent to the surgical field,353 but the extent to which the presence of bacteria near the surgical field influences the development of postoperative SSIs is not clear.354
Proper ventilation, humidity (<68%), and temperature control in the operating room is important for the comfort of surgical personnel and patients, but also in preventing environmental conditions that encourage growth and transmission of microorganisms.355 Operating rooms should be maintained at positive pressure with respect to corridors and adjacent areas.356 Operating rooms typically do not have a variable air handling system. Variable air handling systems are permitted for use in operating rooms only if they continue to provide a positive pressure with respect to the corridors and adjacent areas and the proper ACHs are maintained when the room is occupied. Conventional operating-room ventilation systems produce a minimum of about 15 ACH of filtered air for thermal control, three (20%) of which must be fresh air.120, 357, 358 Air should be introduced at the ceiling and exhausted near the floor.357, 359
Laminar airflow and UVGI have been suggested as adjunct measures to reduce SSI risk for certain operations. Laminar airflow is designed to move particle-free air over the aseptic operating field at a uniform velocity (0.3–0.5 m/sec), sweeping away particles in its path. This air flow can be directed vertically or horizontally, and recirculated air is passed through a HEPA filter.360–363 Neither laminar airflow nor UV light, however, has been conclusively shown to decrease overall SSI risk.356, 364–370
Elective surgery on infectious TB patients should be postponed until such patients have received adequate drug therapy. The use of general anesthesia in TB patients poses infection-control challenges because intubation can induce coughing, and the anesthesia breathing circuit apparatus potentially can become contaminated.371 Although operating room suites at 15 ACH exceed the air exchanges required transmission of TB to operating-room personnel. If feasible, intubation and extubation of the TB surgical patient should be performed in AII. AIA currently does not recommend changing pressure from positive to negative or setting it to neutral; most facilities lack the capability to do so.120 When emergency surgery is indicated for a suspected/diagnosed infectious TB patient, taking specific infection-control measures is prudent (Box 8).
Box 8. Strategy for managing TB patients and preventing airborne transmission in operating rooms*
- If emergency surgery is indicated for a patient with active TB, schedule the TB patient as the last surgical case to provide maximum time for adequate ACH.
- Operating room personnel should use NIOSH-approved N95 respirators without exhalation valves.347
- Keep the operating room door closed after the patient is intubated, and allow adequate time for sufficient ACH to remove 99% of airborne particles (Appendix B, Table B.1.):
- after the patient is intubated and particularly if intubation produces coughing
- if the door to the operating suite must be opened, and intubation induces coughing in the patient; or
- after the patient is extubated and suctioned [unless a closed suctioning system is present].
- Extubate the patient in the operating room or allow the patient to recover in AII rather than in the regular open recovery facilities.
- Temporary use of a portable, industrial grade HEPA filter may expedite removal of airborne contaminants (fresh-air exchange requirements for proper ventilation must still be met).+
- Breathing circuit filters with 0.1–0.2 μm pore size can be used as an adjunct infection-control measure.373, 374
* Material in this table was compiled from references 4, 347, and 372–374.
+ The placement of portable HEPA filter units in the operating room must be carefully evaluated for potential disruptions in normal air flow. The portable unit should be turned off while the surgical procedure is underway and turned on following extubation. Portable HEPA filter units previously placed in construction areas may be used in subsequent patient care, provided that all internal and external surfaces are cleaned and the filter’s performance is verified with appropriate particle testing and is changed, if needed.
Table 10. Summary of ventilation specifications in selected areas of health-care facilities*
| Specifications | AII room (includes bronchoscopy suites) | PE room | Critical care room§ | Isolation anteroom | Operating room |
|---|---|---|---|---|---|
| Air pressure¶ | Negative | Positive | Positive, negative, or neutral | Positive or negative | Positive |
| Room air changes | ≥6 ACH (for existing rooms); ≥12 ACH (for renovation or new construction) |
≥12 ACH | ≥6 ACH | ≥10 ACH | ≥15 ACH |
| Sealed** | Yes | Yes | No | Yes | Yes |
| Filtration supply | 90% (dust-spot ASHRAE 52.1 1992) | 99.97% (Fungal spore filter at point of use (HEPA at 99.97% of 0.3 μm particles)) | >90% | >90% | 90% |
| Recirculation | No (Recirculated air may be used if the exhaust air is first processed through a HEPA filter.) |
Yes | Yes | No | Yes |
* Material in this table is compiled from references 35 and 120.
§ Positive pressure and HEPA filters may be preferred in some rooms in intensive care units (ICUs) caring for large numbers of immunocompromised patients.
¶ Clean-to-dirty: negative to an infectious patient, positive away from an immunocompromised patient.
** Minimized infiltration for ventilation control; pertains to windows, closed doors, and surface joints.
¶¶ Table used with permission of the publisher of reference 35 (Lippincott Williams and Wilkins).
6. Other Aerosol Hazards in Health-Care Facilities
In addition to infectious bioaerosols, several crucial non-infectious, indoor air-quality issues must be addressed by health-care facilities. The presence of sensitizing and allergenic agents and irritants in the workplace (e.g., ethylene oxide, glutaraldehyde, formaldehyde, hexachlorophene, and latex allergens375 ) is increasing. Asthma and dermatologic and systemic reactions often result with exposure to these chemicals. Anesthetic gases and aerosolized medications (e.g., ribavirin, pentamidine, and aminoglycosides) represent some of the emerging potentially hazardous exposures to health-care workers. Containment of the aerosol at the source is the first level of engineering control, but personal protective equipment (e.g., masks, respirators, and glove liners) that distances the worker from the hazard also may be needed.
Laser plumes and surgical smoke represent another potential risk for health-care workers.376–378 Lasers transfer electromagnetic energy into tissues, resulting in the release of a heated plume that includes particles, gases, tissue debris, and offensive smells. One concern is that aerosolized infectious material in the laser plume might reach the nasal mucosa of surgeons and adjacent personnel. Although some viruses (i.e., varicella-zoster virus, pseudorabies virus, and herpes simplex virus) do not aerosolize efficiently,379, 380 other viruses and bacteria (e.g., human papilloma virus [HPV], HIV, coagulasenegative Staphylococcus, Corynebacterium spp., and Neisseria spp.) have been detected in laser plumes.381–387 The presence of an infectious agent in a laser plume may not, however, be sufficient to cause disease from airborne exposure, especially if the normal mode of transmission for the agent is not airborne. No evidence indicated that HIV or hepatitis B virus (HBV) has been transmitted via aerosolization and inhalation.388
Although continuing studies are needed to fully evaluate the risk of laser plumes to surgical personnel, the prevention measures in these other guidelines should be followed:
- NIOSH recommendations,378
- the Recommended Practices for Laser Safety in Practice Settings developed by the Association of periOperative Registered Nurses [AORN],389
- the assessments of ECRI,390–392 and
- the ANSI standard.393
These guidelines recommend the use of
- respirators (N95 or N100) or full face shields and masks,260
- central wall-suction units with in-line filters to collect particulate matter from minimal plumes, and
- dedicated mechanical smoke exhaust systems with a high-efficiency filter to remove large amounts of laser plume.
Although transmission of TB has occurred as a result of abscess management practices that lacked airborne particulate control measures and respiratory protection, use of a smoke evacuator or needle aspirator and a high degree of clinical awareness can help protect healthcare workers when excising and draining an extrapulmonary TB abscess.137
- Page last reviewed: November 5, 2015
- Page last updated: November 5, 2015
- Content source:


 ShareCompartir
ShareCompartir