Water Use in Hemodialysis
Hemodialysis
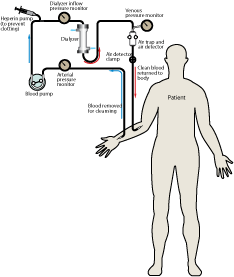
Hemodialysis is a medical procedure for patients who have temporarily or permanently lost kidney function due to renal (kidney) failure. The kidneys are one of the most important organs in the body. They play a vital role in the excretion of waste products from the body, secretion of hormones, and in maintaining the balance of bodily fluids through regulating pH, plasma volume, and blood pressure. Hemodialysis is not a total treatment to replace kidney function as it can not secrete hormones; however it does work to help the body excrete waste and maintain the balance of fluids.
During hemodialysis, blood flows out of the body and by one side of a semi-permeable membrane. Dialysate, the fluid in a dialysis machine, flows by the opposite side of the membrane. Undesired waste in the blood flows into the dialysate, while bicarbonate (a needed solute that helps in pH balance) flows from the dialysate into the blood. The clean blood is then returned to your body. Removing the harmful waste and extra salt and fluids helps control blood pressure, pH balance, and plasma volume, similar to the results of a functioning kidney.
For the health and safety of hemodialysis patients, it is vital to ensure that the water that is used to make dialysate is safe and clean. According to CDC's Division of Healthcare Quality Promotion and the Healthcare Infection Control Practices Advisory Committee (HICPAC), hemodialysis requires "special water-treatment processes to prevent adverse patient outcomes of dialysis therapy resulting from improper formulation of dialysate with water containing high levels of certain chemical or biological contaminants. The Association for the Advancement of Medical Instrumentation (AAMI) has established chemical and microbiologic standards for the water used to prepare dialysate, substitution fluid, or to reprocess hemodialyzers for renal replacement therapy. The AAMI standards address: a) equipment and processes used to purify water for the preparation of concentrates and dialysate and the reprocessing of dialyzers for multiple use and b) the devices used to store and distribute this water (1)."
For more information on the rationale for water treatment in hemodialysis, visit Guidelines for Environmental Infection Control in Health-Care Facilities [PDF - 4.99 mb] (250 pages) from CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC).
More information on hospital-based recommendations for medical uses of water is found at CDC’s Infection Control Guidelines (Division of Healthcare Quality Promotion).
Recommendations for Dialysis Water Quality and Dialysate
Guidelines for Environmental Infection Control in Health Care Facilities-Dialysis Water Quality and Dialysate
Recommendations of CDC's Division of Healthcare Quality Promotion and the Healthcare Infection Control Practices Advisory Committee (HICPAC).
[This information was taken directly from page 19 of the MMWR report that can be found at http://www.cdc.gov/mmwr/PDF/rr/rr5210.pdf [PDF - 1.2 mb] ]
- Adhere to current AAMI standards for quality assurance performance of devices and equipment used to treat, store, and distribute water in hemodialysis centers (both acute and maintenance [chronic] settings) and for the preparation of concentrates and dialysate. Category IA, IC
- No recommendation is offered regarding whether more stringent requirements for water quality should be imposed in hemofiltration and hemodiafiltration. Unresolved issue
- Conduct microbiological testing specific to water in dialysis settings. Category IA, IC
- Perform bacteriologic assays of water and dialysis fluids at least once a month and during outbreaks using standard quantitative methods. Category IA, IC
- Assay for heterotrophic, mesophilic bacteria (for example, Pseudomonas spp.).
- Do not use nutrient-rich media (for example, blood agar or chocolate agar).
- In conjunction with microbiological testing, perform endotoxin testing on product water used to reprocess dialyzers for multiple use. Category IA, IC
- Ensure that water does not exceed the limits for microbial counts and endotoxin concentrations outlined in Table 18. Category IA
- Perform bacteriologic assays of water and dialysis fluids at least once a month and during outbreaks using standard quantitative methods. Category IA, IC
- Disinfect water distribution systems in dialysis settings on a regular schedule. Monthly disinfection is recommended. Category IA, IC
- Whenever practical, design and engineer water systems in dialysis settings to avoid incorporating joints, dead-end pipes, and unused branches and taps that can harbor bacteria. Category IA, IC
- When storage tanks are used in dialysis systems, they should be routinely drained, disinfected with an EPA-registered product, and fitted with an ultrafilter or pyrogenic filter (membrane filter with a pore size sufficient to remove small particles and molecules >1 kilodalton) installed in the water line distal to the storage tank. Category IC
Rating Categories
Recommendations are rated according to the following categories:
- Category IA
- Strongly recommended for implementation and strongly supported by well-designed experimental, clinical, or epidemiologic studies.
- Category IB
- Strongly recommended for implementation and supported by certain experimental, clinical, or epidemiologic studies and a strong theoretic rationale.
- Category IC
- Required by state or federal regulation, or representing an established association standard. (Note: Abbreviations for governing agencies and regulatory citations are listed where appropriate. Recommendations from regulations adopted at state levels are also noted. Recommendations from AIA guidelines cite the appropriate sections of the standards.)
- Category II
- Suggested for implementation and supported by suggestive clinical or epidemiologic studies, or a theoretic rationale.
- Unresolved issue
- No recommendation is offered. No consensus or insufficient evidence exists regarding efficacy.
References
- CDC and HICPAC. Guidelines for Environmental Infection Control in Healthcare Facilities. Available at http://www.cdc.gov/ncidod/dhqp/pdf/guidelines/Enviro_guide_03.pdf. [PDF - 1.4 mb].
Related Links
Here at CDC
- Infection Control Guidelines
- Prevention and Control of Dialysis Associated Infections
- Renal Dialysis Units during a Boil Water Advisory
- Emergency Operations (includes safe use of tanker water, boil water alerts, etc)
CDC and the Healthcare Infections and Control Practices Advisory Committee (HICPAC)
U.S. Department of Health & Human Services
Around the Web (Non-governmental)
- Dialysis Standards Collection (Association for the Advancement of Medical Instrumentation)
- Page last reviewed: October 11, 2016
- Page last updated: October 11, 2016
- Content source:


 ShareCompartir
ShareCompartir