An Update on Global Partners Reaching the Ultimate Goal of Eliminating Epidemic Meningitis as a Public Health Concern in Africa
Related Links
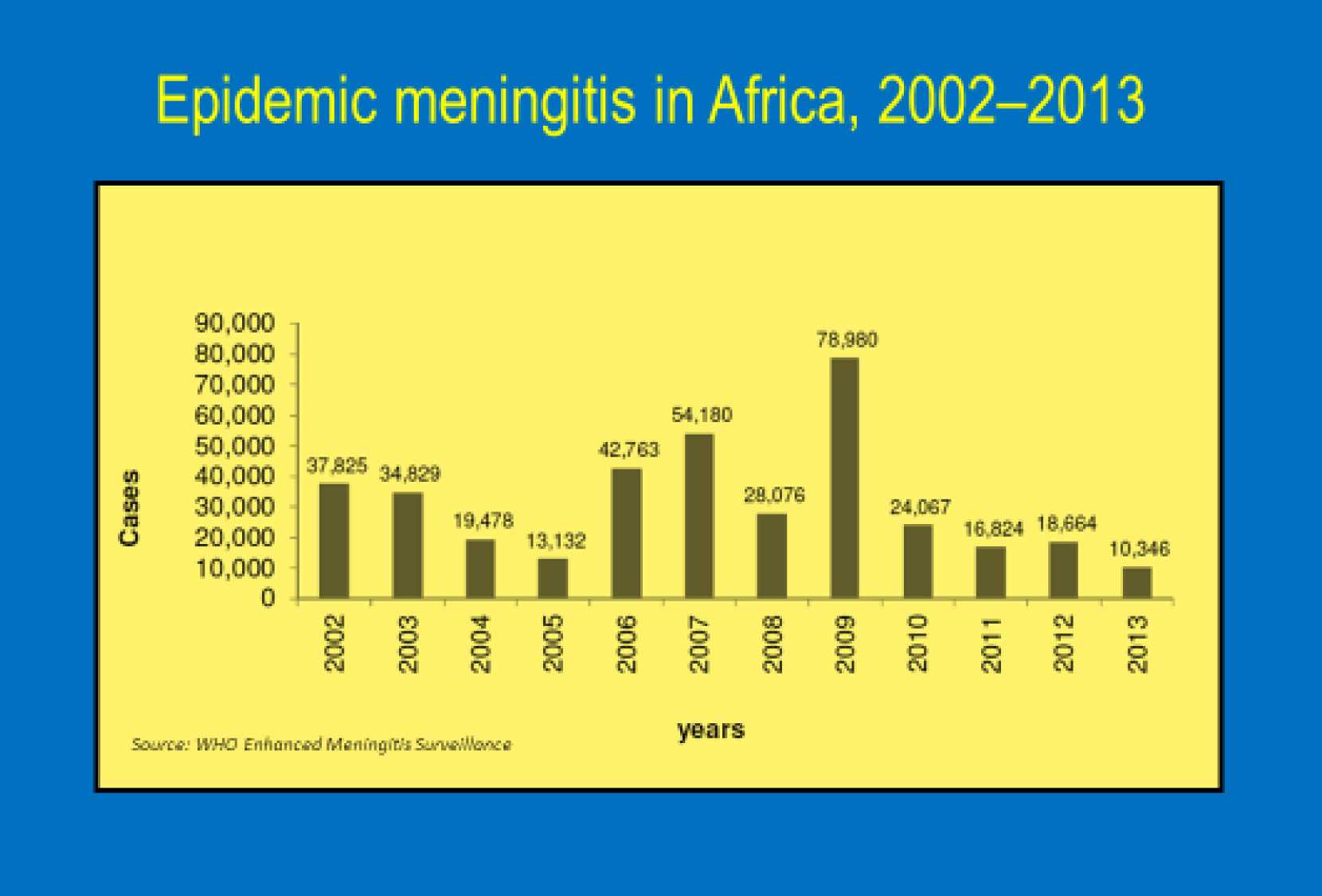
Worldwide, the largest burden of meningococcal meningitis (caused by Neisseria meningitidis) occurs in the sub-Saharan Africa meningitis belt— from Senegal in the west to Ethiopia in the east where an estimated 430 million people are at risk from meningitis epidemics.About 90% of meningitis cases in this region are due to serogroup A. Meningococcal meningitis is a highly contagious infection of the linings that surround the brain and spinal cord. It can cause severe brain damage and is fatal in 50% of cases if untreated. The human and socioeconomic toll of these epidemics are devastating.
The Centers for Disease Control and Prevention (CDC) has a long history of working with global partners to fight meningitis in Africa. Over the years, agency staff have frequently made the 20-hour journey to West Africa to work alongside local health officials and global partners to build and sustain local capacity to fight this disease, while CDC staff in Atlanta, Georgia USA have conducted meningitis research, multifaceted testing and vaccine validation studies.
A New Vaccine for the Meningitis Belt
A 2008 commitment by the African Ministers of Health to eliminate serogroup A Meningitis epidemics issued through the Yaounde Declaration paved the way for roll-out of the new vaccine—MenAfriVac ®. The Bill & Melinda Gates Foundation and consortium of global partners funded the affordable serogroup A conjugate vaccine developed by The Meningitis Vaccine Project specifically for Africa—and pushed for an unprecedented quick timeline moving the vaccine from licensure in December 2009 to the first mass vaccination campaign in Burkina Faso in the fall of 2010. Researchers believed this vaccine, priced at just under $.50 per dose and targeted to persons 1 to 29 years of age would provide high immunogenicity, particularly for children under age two, longer duration of protection, and possible “herd” immunity. Since 2010, vaccination campaigns for MenAfriVac® have been conducted in Benin, Burkina Faso, Cameroon, Chad, Côte d'Ivoire, Ethiopia, Ghana, Mali, Mauritania, Niger, Nigeria, Senegal, Sudan, Togo, and The Gambia. 217 million people in these 15 countries have been vaccinated with no reported vaccine failures and no serious adverse reactions. Researchers have shown that Serogroup A Meningitis epidemics are being eliminated by these mass campaigns and there is an all-time low disease burden with early evidence of herd immunity. In January, the World Health Organization (WHO) paved the way toward sustaining this herd immunity by approving use of MenAfriVac® for routine immunization of infants in sub-Saharan Africa which will protect millions of children at risk of meningitis.

In 2010, just prior to the first full scale vaccination campaign using the meningococcal A conjugate vaccine MenAfriVac® , Fabien Diomandé, MD, MSc, medical epidemiologist (as CDC secondee) and Ryan Novak, PhD, as the epidemiologist/coordinator for Africa meningitis activities, walked along railroad tracks in rural Burkina Faso to deliver vaccine to a local vaccination center for a pilot safety evaluation study.
A New Global Partnership Undertakes Surveillance and Evaluation
Because it is essential to evaluate the impact of a vaccine following introduction, WHO recommended implementation of case based surveillance to monitor the impact of MenAfriVac® and meningococcal serogroup trends. While clinical trials for the vaccine demonstrated it to be extremely safe and immunogenic—this was the first real-world experience using this vaccine at a public health scale.
In October 2013, through the CDC Foundation, the Bill and Melinda Gates Foundation awarded a five-year $10 million grant to CDC to launch MenAfriNet—a regional meningitis surveillance network to evaluate the impact of MenAfriVac® in the African meningitis belt. CDC is leading MenAfriNet in partnership with WHO-AFRO, Agence de Médicine Préventive, African Ministries of Health, and non-governmental agencies with meningitis surveillance expertise to collect and analyze high quality case-based meningitis surveillance data. Built on the Integrated Disease Surveillance and Response (IDSR) platform, MenAfrNet activities have strengthened data collection for other IDSR conditions such as measles, and viral hemorrhagic fever.
Activities in the comprehensive MenAfriNet approach range from evaluating the impact of MenAfriVac®, guiding research on this vaccine’s long-term effectiveness, herd immunity, serogroup replacement, as well as informing decision-making regarding future vaccines. Investment in surveillance and operational research such as coverage surveys ensure that these effective MenAfriVac® vaccination programs are sustained. CDC’s activities focus on vaccine impact on disease burden, carriage and strengthening local surveillance and laboratory capacity. Burkina Faso, Mali, Niger and Togo are currently participating in MenAfriNet and plans are underway to integrate additional countries, possibly Chad and Ethiopia.
Continuously Strengthening Local Capacity to Fight Meningitis

Partners from WHO, WHO-AFRO, WHO-IST, Agence de Médicine Préventive, Ministries of Health,non-governmental agencies and CDC staff at 1st Annual MenAfriNet Partners Meeting in Burkina Faso, February 2014
Since MenAfriNet’s launch, CDC has hosted trainings at its campus in Atlanta, Georgia USA for public health practitioners involved in MenAfriNet, including data managers from Burkina Faso, the WHO Africa Regional Office and the WHO Intercountry Support Team for West Africa as well as a leading laboratory quality assurance expert from Agence de Médecine Préventive who worked with CDC’s international Meningitis laboratory team to initiate the laboratory working group to support laboratory strengthening activities in Burkina Faso, Mali, Togo, and Niger.
CDC partnered with the WHO Inter-Country Support Team for West Africa to host 48public health professionals from Burkina Faso, Mali, Niger, Togo, WHO, AMP and CDC for a MenAfriNet Surveillance and Data Management Workshop in Burkina Faso in March of this year. During the workshop, p participants revised the Generic Guidelines for Case-Based Surveillance of Bacterial Meningitis in the WHO African Region and were trained in the MenAfriNet Epi Info 7 Data Management System in order to implement the system in their home countries prior to the next meningitis season. More in-country capacity strengthening activities are planned for later this year.
Demonstrating Public Health Scale Effectiveness: Burkina Faso
A principal goal of the Burkina Faso introduction of MenAfriVac® was development of herd immunity. Despite its being a resource-limited setting, high-quality national surveillance was achieved in Burkina Faso and the impact of MenAfriVac® was demonstrated, providing the first data validating its effectiveness on a public health scale. In addition, a major effort was made to study serogroup A meningitis circulation in the Burkina Faso general population before and after the introduction of MenAfriVac® and to conduct carriage studies. Researchers say, that the observation of disease reduction in the population broadly, not only among vaccine recipients, suggests that there has been an interruption of carriage and transmission of serogroup A meningitis due to herd immunity, though questions remain regarding the duration of protection and strength of herd immunity without integration into routine immunization programs (EPI).
Going Forward
As our nation moves to accelerate progress toward a world safe and secure from infectious disease threats through its Global Health Security agenda, President Obama has said, “…we must come together to prevent, and detect, and fight every kind of biological danger—whether it’s a pandemic like H1N1, or a terrorist threat, or a treatable disease.” Partners from around the world have come together to tackle serogroup A meningitis in sub-Saharan Africa—and their rapid, effective multi-sectorial responses, international coordination and culturally sensitive communication strategies have been and remain paramount to ending these epidemics and the associated devastating disabilities, loss of life, instability, and economic upheaval they cause. The potential impact of MenAfriVac® promises awe-inspiring outcomes: 142,000 deaths could be prevented, permanent disabilities could be prevented in 284,000 children and adults and a savings of approximately $99.7 million in direct medical costs could be realized in 10 years within the seven most affected countries of the meningitis belt.
In observance of World Meningitis Day 2015, we can proudly recognize how the shared vision and on-going commitment of global partners is eliminating serogroup A meningitis as a public health concern in Africa. It took ten long years to develop and deliver the lifesaving MenAfriVac® to Sub-Saharan Africa. Now, all 26 African countries considered at risk for serogroup A meningitis epidemics have plans to introduce the vaccine by 2016. We know that introduction alone will not be enough. Much more science, program and policy work will be required to evaluate the vaccine’s effectiveness. Partners must continue to help: strengthen local capacity, fully integrate the vaccine into the routine childhood immunization schedules and provide technical support to replicate this successful model for other diseases. Learn more about how CDC continues to foster global partnerships to fight meningitis in Africa at: http://www.cdc.gov/meningococcal/global.html .
- Page last reviewed: April 23, 2015
- Page last updated: April 23, 2015
- Content source:
Global Health
Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.


 ShareCompartir
ShareCompartir