Eliminate Hepatitis: The Importance of Hepatitis B Vaccine Birth Dose
Globally, hepatitis B virus (HBV), the major cause of liver cancer and the 7th leading cause of death worldwide, affects approximately 257 million persons.[1],[2] HBV is a vaccine-preventable disease, and hepatitis B vaccination remains the most effective and cost-saving strategy to prevent infection and its long-term consequences. The birth dose of the hepatitis B vaccine is especially important, since chronic HBV infection develops in 90% of infants infected before 1 year of age.[3] The hepatitis B vaccine is most effective at preventing mother-to-child transmission when the first dose is given within 24 hours after birth, followed by 2-3 additional doses later. In the absence of the universal birth dose, the transmission of HBV infection from mother to child remains a major source of chronic liver disease when infected children become adults.[4] The birth dose costs only $0.20, which makes it one of the cheapest vaccines available on the market.[5]
CDC provides direct technical and financial assistance to the World Health Organization and to countries’ immunization programs to decrease the burden of hepatitis B virus infection. CDC also has staff assigned to World Health Organization (WHO) Regional Offices to support hepatitis B vaccination activities directly.
Thinking outside of “the box”—outside the cold chain
Like many vaccines, the recommended storage conditions for the hepatitis B vaccine are at temperatures ranging from 2-8°C to prevent it from becoming ineffective. In several countries in Asia and Africa, the majority of babies are born at home or in health facilities that do not have reliable sources of electricity or working refrigerators, making it a challenge to vaccinate babies with hepatitis B vaccine birth dose within 24 hours of birth. Evidence indicates that the hepatitis B vaccine birth dose can be kept outside refrigeration (also known as outside the cold chain or in controlled temperature chain) up to a month without losing its potency if temperatures do not exceed 37°C.[6]
Based on this evidence, CDC supported pilot projects in several countries to determine if this intervention improves hepatitis B birth dose vaccination rates. In the Solomon Islands, storing the hepatitis B vaccine outside the cold chain led to a 500% increase in hepatitis B vaccination coverage among children born at home.[7] Because of this success and with further support from the CDC, the Solomon Islands Ministry of Health approved wider implementation in other areas, based on the lessons learned from the pilot study. Similar innovative projects were implemented in Lao[8],[9] and Kiribati[10] and increased hepatitis B vaccine birth dose vaccination.
Making a case for birth dose immunizations
As of 2016, only 98 (50%) countries had introduced the recommended universal hepatitis B vaccine birth dose. A substantial burden of chronic HBV infection persists because global coverage with the birth dose is still low (39%). Despite the WHO recommendation to vaccinate every child with a birth dose of the hepatitis B vaccine, several countries require data showing the burden of disease among children prior to introducing the birth dose. Other countries need this data to evaluate their hepatitis B vaccination program and make improvements where needed.
To meet this need, CDC supported blood testing (serosurveys) in the Solomon Islands, which revealed areas that need to improve their hepatitis B vaccination coverage to be able to achieve the regional control goal of <1% prevalence of chronic HBV infection among children. CDC also worked with the WHO Africa Regional Office to document the progress in hepatitis B control through vaccination in the region.[11] Only 11 of 47 countries provide hepatitis B vaccine birth dose in Africa despite the high burden. CDC suggested strategies that were successful in other regions that could be used in Africa to vaccinate children as soon as possible after birth. In 2017, CDC will be conducting a serosurvey in Haiti to document the burden of hepatitis B among children and gather evidence on the need to introduce the birth dose.
Setting goals and verifying their achievement
In 2016, the 69th World Health Assembly endorsed the Global Health Sector Strategy on Viral Hepatitis, which calls for elimination by 2030. To meet this goal, each WHO Region established specific hepatitis B goals to achieve by certain deadlines. Verification of achievement of those goals is measured by conducting nationally representative serosurveys of hepatitis B infection in children. In 2016, CDC provided technical support to Cambodia and Lebanon to implement nationally representative serosurveys among children to verify the achievement of hepatitis B control. CDC is also working with the Pan American Health Organization to implement an innovative cost-saving serosurvey approach to verify the elimination of hepatitis B infection in countries in the Americas.
A brighter future
Since most of the hepatitis B infections in the United States are acquired overseas, the efforts by CDC not only protect the United States from further increase in hepatitis B and liver cancer rates, but also help achieve elimination of viral hepatitis in the United States. Future projects include estimating the burden of mother-to-child transmission of HBV infection in Africa to inform birth dose introduction policy in the African Region; supporting hepatitis B vaccine birth dose improvement activities in Vietnam; supporting hepatitis B serosurveys in Colombia, Philippines, and Fiji to verify achievement of regional control/elimination goals; and supporting the establishment of hepatitis B control goal verification guidelines in the Eastern Mediterranean and European Regions.
In the Western Pacific Region alone, with commitment from WHO Member States and CDC support, the hepatitis B infection burden decreased from over 8% in the 1990s to less than 1% in 2014 among children.[12] CDC will continue to provide technical and financial support to introduce the birth dose and improve hepatitis B vaccination globally.
[1]World Health Organization. Global Hepatitis Report, 2017. Available at: http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?ua=1
[2] GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;385:117–71.
[3] WHO. Hepatitis B Vaccines: WHO position paper – July 2017. Weekly Epidemiological Record 2017; 92: 369-392.
[4] WHO. Hepatitis B Vaccines: WHO position paper – July 2017. Weekly Epidemiological Record 2017; 92: 369-392.
[5] https://www.unicef.org/supply/files/HepB.pdf
[6] https://www.path.org/publications/files/TS_opt_occ_lit_rev.pdf
[7] Breakwell et al. Evaluation of storing hepatitis B vaccine outside the cold chain in the Solomon Islands: identifying opportunities and barriers to implementation. Vaccine 2017; 25: 2770-2774.
[8] Kolwaite AR, Xeuatvongsa A, Ramirez-Gonzalez A, Wannemuehler K, Vongxay V, Vilayvone V, Hennessey K, Patel MK. Hepatitis B vaccine stored outside the cold chain setting: a pilot study in rural Lao PDR.
Vaccine. 2016 Jun 14;34(28):3324-30.
[9] Xeuatvongsa A, Datta SS, Moturi E, Wannemuehler K, Philakong P, Vongxay V, Vilayvone V, Patel MK. Improving hepatitis B birth dose in rural Lao People’s Democratic Republic through the use of mobile phones to facilitate communication. Vaccine. 2016 Nov 11;34(47):5777-5784
[10] Li X, Heffelfinger J, Wiesen E, Diorditsa S, Valiakolleri J, Nikuata AB, Nukuro E, Tabwaia B, Woodring J. Improving hepatitis B birth dose coverage through village health volunteer training and pregnant women education. Vaccine. 2017 Jul 5. pii: S0264-410X(17)30853-8. doi: 10.1016/j.vaccine.2017.06.056. [Epub ahead of print]
[11] Breakwell, et al. The Status of Hepatitis B Control in the African Region. The Pan African Medical Journal. 2017;27 (Supp 3):17. doi:10.11604/pamj.supp.2017.27.3.11981
[12] Wiersen E, et al. Progress towards hepatitis B prevention through vaccination in the Western Pacific, 1990-2014. Vaccine 2016; 34: 2855-2862

Immunization staff from different provinces in Solomon Islands reviewing their hepatitis B birth dose coverage data after implementation of OCC strategy

Children waiting to get tested for hepatitis B in school-based survey in the Solomon Islands
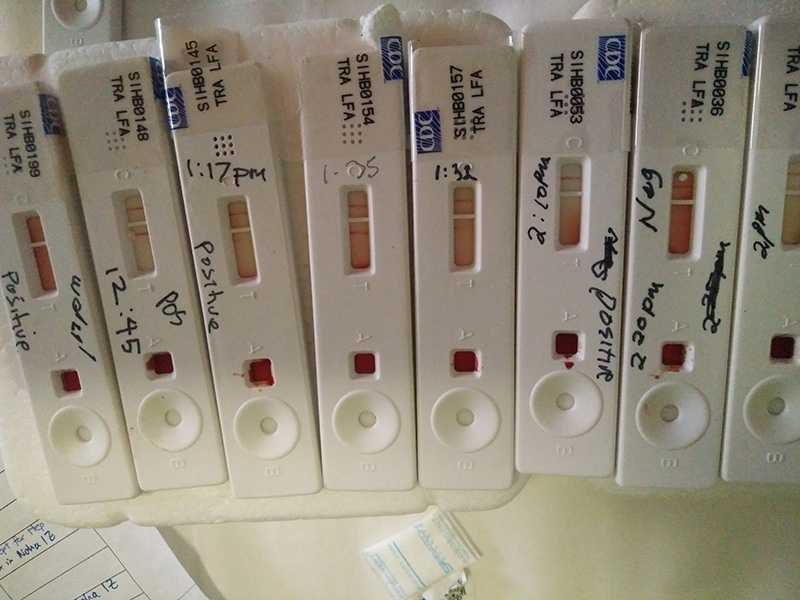
Hepatitis B surface antigen rapid tests conducted in the Solomon Islands during a serosurvey among school-aged children
- Page last reviewed: July 27, 2017
- Page last updated: July 27, 2017
- Content source:
Global Health
Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.


 ShareCompartir
ShareCompartir