Culture-Independent Diagnostic Tests
What are CIDTs and How Do They Affect Foodborne Illness?
CIDTs allow doctors to rapidly determine the cause of a patient’s illness. However, these tests do not provide the information needed to find and prevent foodborne illness outbreaks and monitor disease trends.
 Culture-independent diagnostic tests (CIDTs) are changing the way that clinical laboratories diagnose patients with foodborne illness. These tests can identify the general type of bacteria causing illness within hours, without having to culture, or grow the bacteria in a laboratory. (The pure bacterial strain that grows is called an isolate.) These tests allow doctors to rapidly determine the cause of a patient’s illness. However, a shift toward CIDTs means that clinical laboratories may stop culturing, or producing bacterial isolates, from patients with foodborne illness. For example, the percentage of Campylobacter diarrheal illnesses diagnosed only by CIDTs in FoodNet sites increased from 13% in 2012-2014 to 24% in 2015.
Culture-independent diagnostic tests (CIDTs) are changing the way that clinical laboratories diagnose patients with foodborne illness. These tests can identify the general type of bacteria causing illness within hours, without having to culture, or grow the bacteria in a laboratory. (The pure bacterial strain that grows is called an isolate.) These tests allow doctors to rapidly determine the cause of a patient’s illness. However, a shift toward CIDTs means that clinical laboratories may stop culturing, or producing bacterial isolates, from patients with foodborne illness. For example, the percentage of Campylobacter diarrheal illnesses diagnosed only by CIDTs in FoodNet sites increased from 13% in 2012-2014 to 24% in 2015.
Without a bacterial isolate from the culturing process, public health scientists can’t perform tests that determine an organism’s strain or subtype (such as DNA fingerprints), resistance pattern, or other characteristics. This information is needed to detect and prevent outbreaks, track antibiotic resistance, and monitor disease trends to know if prevention measures are working.
How Do CIDTs Affect the Ability of Public Health Officials to Detect and Prevent Outbreaks?

A microbiologist is using whole genome sequencing to determine the DNA fingerprint of the bacteria that made someone sick.
- PulseNet, a laboratory network that connects foodborne illness cases to detect outbreaks, extracts important information from the bacteria that are found in samples from sick people.
- PulseNet needs bacterial isolates to determine the precise strains making people ill, using DNA fingerprinting methods, including a new precise method known as whole genome sequencing (WGS).
- Public health officials look at PulseNet data for clusters of matching DNA fingerprints. If epidemiologists find that the cluster has a common source, it is called an outbreak.
- CIDTs skip the step of producing an isolate and as a result, DNA fingerprints cannot be produced for PulseNet. Without DNA fingerprints from the bacteria, PulseNet won’t be able to continue detecting clusters.
- Without PulseNet cluster detection, outbreaks may not even be recognized, contaminated products may remain on shelves and in pantries, more people may become sick, and valuable opportunities for improving the safety of our food may be lost.
- PulseNet prevents an estimated 270,000 illnesses every year and saves about $507 million every year in medical costs and lost productivity.
How Do CIDTs Affect the Ability to Track and Contain Antibiotic Resistance?
- Public health officials gather information about antibiotic resistance by analyzing the bacteria isolates after they are grown in a culture.
- If cultured bacterial isolates are not available to be analyzed, we will not know how resistant they are to antibiotics. This may affect how the illness is treated and make it difficult to monitor trends in resistance over time.
- Monitoring antibiotic resistance sometimes results in regulatory action to protect health, such as the ban of fluoroquinolone use in poultry after scientific data showed its use was causing an increase in antibiotic-resistant Campylobacter, which cause foodborne illness.
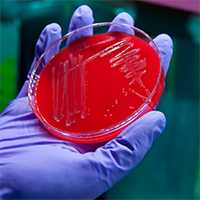
This image shows the final stage of the bacterial culture process. Clumps of pure bacteria, or bacterial isolates, are growing on a culture or petri dish.
How Do CIDTs Affect the Ability to Monitor Disease Trends?
- CIDTs may improve our ability to monitor certain types of disease trends, such as diseases caused by bacteria, viruses, or parasites for which practical tests were formerly not available.
- CIDTs do not produce bacterial isolates that are needed to distinguish between strains and subtypes of bacteria. Therefore, public health officials are not able to track important changes in rates of illnesses caused by specific types of bacteria.
- This also limits the ability to detect, investigate, and control multistate outbreaks.
- Regulatory agencies and industries need the information from outbreak investigations and disease trends to take actions to make food safer.
- Currently, only culture-confirmed infections are counted in tracking foodborne illness trends. Therefore, the trend data do not include infections that are CIDT positive but not culture-confirmed.
Potential Short-Term Solutions
- CDC is encouraging clinical laboratories to work with public health laboratories to continue culturing and isolating the harmful bacteria from ill people with positive CIDTs. Patient specimens with a positive CIDT for Salmonella, Shiga toxin-producing E. coli, and Shigella should be cultured to isolate the bacterial strain. Selected laboratories should also do this for Campylobacter.
- CDC is considering ways to make follow-up cultures easier and cheaper for clinical laboratories.
- CDC is working closely with the Association of Public Health Laboratories (APHL), public health officials, regulatory agencies, diagnostic laboratories, CIDT kit manufacturers, and clinicians to make sure that cultures are obtained in clinical labs when CIDTs are positive, or the positive specimen from the patient is provided to public health laboratories so they can culture it.
- CDC is encouraging companies that make CIDTs to design the tests in a way that keeps the bacteria alive so they can be cultured if the test is positive.
- CDC is adapting surveillance systems like the Foodborne Diseases Active Surveillance Network, or FoodNet, to include infections diagnosed only by CIDTs.
Potential Long-Term Solutions
- CDC is working with partners to develop advanced testing methods that will not require bacterial isolates to provide information needed by public health officials. These tests may also provide additional information to healthcare providers about the germ’s potential for antibiotic resistance or likelihood of causing serious illness.
These innovations are in the early stages of development and could be years away from implementation. Researchers hope that the use of these methods will eventually eliminate the need for culturing bacterial isolates from most patients and speed up the process of detecting and solving outbreaks. Culture-derived bacterial isolates from patients will still be needed for a long time to identify emerging problems.
Related Publications
- “Infection with Pathogens Transmitted Commonly Through Food and the Effect of Increasing Use of Culture-Independent Diagnostic Tests on Surveillance — Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2012–2015”: Morbidity and Mortality Weekly Report
- “An Economic Evaluation of PulseNet”: American Journal of Preventive Medicine
- “Implementation of Nationwide Real-time Whole-genome Sequencing to Enhance Listeriosis Outbreak Detection and Investigation”: Clinical Infectious Diseases
- “Effect of Culture-Independent Diagnostic Tests on Future Emerging Infections Program Surveillance”: Emerging Infectious Diseases journal article
- “Bacterial Enteric Infections Detected by Culture-Independent Diagnostic Tests- FoodNet, United States, 2012-2014”: Morbidity and Mortality Weekly Report
- Page last reviewed: July 30, 2016
- Page last updated: July 30, 2016
- Content source:


 ShareCompartir
ShareCompartir