2011-2012 Flu Season Draws to a Close
Last full FluView posted May 25, 2012 Shows U.S. Influenza Activity at Summer Levels
A Late and Mild Flu Season
May 25, 2012 – Today the Centers for Disease Control and Prevention issued the final full influenza surveillance report for the 2011-2012 season. The report – titled “FluView” – shows that influenza activity in the United States is minimal across most of the country, wrapping up a season that began late and was mild compared to most previous seasons for which surveillance data is available. In fact, the season set a new record for the lowest and shortest peak for influenza-like-illness since this type of surveillance began.
Influenza-like-illness (ILI) in the United States typically begins to increase in late December or early January and peaks in February most commonly. This season, ILI remained low through February and did not reach baseline until mid-March. ILI never exceeded baseline during the season. According CDC’s Dr. Joseph Bresee, “This is the first time since CDC started this kind of influenza-like-surveillance (ILI) that the percentage of patient visits for ILI was elevated for only one week of the season.” Dr. Bresee is Chief of the Epidemiology and Prevention Branch in CDC’s Influenza Division. In past seasons, ILI has remained above baseline for between 8 and 20 weeks, with an average of 13 weeks at or above baseline each season since this type of surveillance began in 1997-1998. Bresee adds, “In terms of ILI, this not only the shortest time we were above baseline, but it’s also the lowest ‘peak’ ever recorded.” The graph below compares ILI from five different seasons, including the current season (2011-2012), the 2009 H1N1 pandemic season, a ‘moderately severe’ flu season (2007-2008), as well as a season classified as ’moderate’ in severity (2002-03).
For a more detailed view of the graphic, please click on the image or visit ILI Weekly National Summary detail.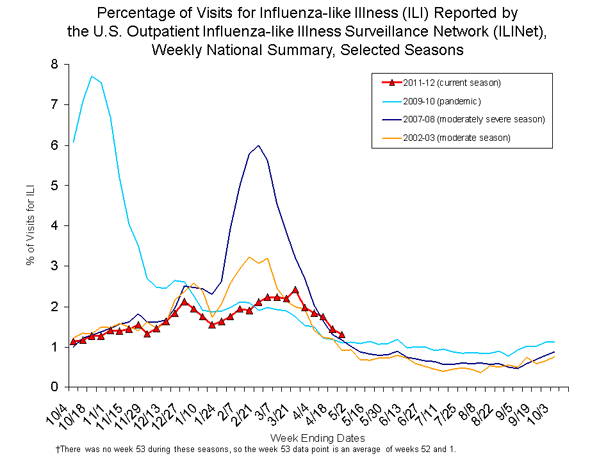
ILI nationally was low despite the fact that high percentages of respiratory specimens tested positive for influenza in parts of the country from early February until late May, and even then, the percent of specimens testing positive for flu remained above 10% for the Week 20 report. High percentages of respiratory specimens testing positive for flu usually mean high flu activity or flu outbreaks and historically, the 10% mark has been used as an informal marker for the beginning and end of the flu season. This season, improved screening and testing procedures at labs may have contributed to higher levels of percent positive tests than have been seen previously. It’s also possible that the lateness of the season contributed to higher levels of specimens testing positive for flu for longer. According to Bresee, “With little else circulating that would result in influenza-like-illness, it would make sense that we would find a higher proportion of flu viruses since that is pretty much all that is out there right now.” CDC epidemiologists will be looking at this indicator closely over the next couple of years to see whether a “new normal” for percent of respiratory specimens testing positive for flu may have emerged as a result of laboratory and testing improvements. Nevertheless, according to Bresee “the high numbers of percent positive specimens that we saw this season were indicative of flu activity. While these localized influenza outbreaks occurred from February through late May, they were never prolonged or extensive enough to raise the national level of ILI substantially.”
Other key flu indicators were low this season as well. As of May 25, 2012, 26 pediatric deaths occurring during the 2011-2012 season had been reported to CDC; this is the lowest number of pediatric deaths reported during a season since such record-keeping began. These are deaths in children younger than 18 who test positive for influenza. These deaths have been legally reportable by states to CDC since 2004. Since that time, the number of pediatric deaths has ranged from a low of 46 deaths during the 2005-2006 season, to a high of 282 deaths reported during the 2009-2010 season, which included pediatric deaths occurring during the 2009 H1N1 pandemic. It’s possible that additional deaths will be reported for the 2011-2012 season as delays in reporting sometimes occur and surveillance for pediatric mortality occurs year-round.
Top of PageInteractive Web Tools
A new interactive web application in “FluView,” displays information – from this season and previous season’s – related to flu-related pediatric deaths. Other interactive tools display ILI over time and ILI and laboratory data on a national and regional level over time.
Why Was Flu So Late and Mild This Year
According to CDC’s Bresee, “The reason for the mildness and lateness of the season isn’t certain, but it’s likely that there were a number of contributing factors, including a mild winter, the fact that most of the influenza viruses circulating this season were similar to those that have circulated for the past two seasons and the fact that most circulating viruses were similar to the viruses that the 2011-2012 vaccine was designed to protect against.” The low levels of influenza virus “drift” (change) for two consecutive years and “steadily increasing influenza vaccination coverage in the country likely contributed to broad levels of immunity in the population,” says Bresee.
Vaccine Match & Vaccine Effectiveness (VE)
How well the flu vaccine works (or its ability to prevent influenza illness) can range widely from season to season and also can vary, depending largely on two factors:1) the characteristics of the person being vaccinated (such as their age and health), and 2) the similarity or "match" between the influenza viruses in the vaccine and those spreading in the community. The closer the match, the better the vaccine is likely to be in preventing influenza illness. If the viruses in the vaccine are very different from circulating influenza viruses, vaccine effects can be lower. However, even during years when the vaccine match is very good, the benefits of vaccination will vary across the population, depending on host factors like the health and age of the person being vaccinated, for example. In general, the flu vaccine works best among young healthy adults and older children. Lesser effects of flu vaccine are often found in studies of young children (e.g., those younger than 2 years of age) and older adults. Older people with weaker immune systems often have a lower protective immune response after influenza vaccination compared to younger, healthier persons. This can result in lower levels of vaccine effectiveness in these people. According to Bresee, “When combined, match and host factors mean that no influenza vaccine made now will ever be 100% effective.”
During the 2011-2012 season, influenza A (H3N2), 2009 influenza A (H1N1), and influenza B viruses co-circulated in the United States. Over the course of the season, predominant viruses varied from region to region and between states, but nationally, influenza A (H3N2) influenza viruses predominated. Most of the viruses tested this season were well-matched to the vaccine viruses (the viruses the vaccine is designed to protect against).
CDC works with researchers at universities and hospitals to estimate how well influenza vaccine works through observational studies using laboratory-confirmed influenza as the outcome. CDC’s studies are conducted in 5 sites across the United States to gather representative data. To assess how well the vaccine works across different age groups, CDC’s studies include all people aged 6 months and older recommended for an annual influenza vaccination. A preliminary assessment of vaccine effectiveness (VE) based on data collected from January 2012 through March 2012, found VE of 52% (95% CI 38% to 63%), after adjusting for study site, date of symptom onset, age group, race, days between symptom onset and testing, and health status (very good/excellent vs. not). Data collection for this study continued through April. In addition to collecting data later in the 2011-2012 season, the final dataset will include information collected from medical records, including verification of vaccine receipt.
According to Bresee, the results of the interim data analysis for the 2011-2012 season are fairly consistent with results from the 2010-2011 influenza season, during which circulating viruses and those in the vaccine also were well matched. Results from that season indicated that influenza vaccine effectiveness was about 60% overall. “We know flu vaccine isn’t perfect. It isn’t the best tool in an ideal world, but it is the best tool we have right now to protect against the flu. We are working on figuring out how to make vaccine faster; how to make vaccine that works better in the elderly; and how to make vaccine that cross-protects against different flu viruses, but in the meantime, we need to use the tool we have at our disposal, to protect as many people as we can,” Bresee concludes.
Manufacturers reported distributing 132 million doses of influenza vaccine in the United States during the 2011-2012 season.
More information about the 2011-2012 flu season and the composition of the 2012-2013 seasonal flu vaccine is available in the Morbidity and Mortality Weekly Report (MMWR) Update: Influenza Activity — United States, 2011–12 Season and Composition of the 2012–13 Influenza Vaccine.
Top of Page- Page last reviewed: May 25, 2012
- Page last updated: June 14, 2012
- Content source:
- Centers for Disease Control and Prevention
- Page maintained by: Office of Associate Director of Communication, Division of Public Affairs


 ShareCompartir
ShareCompartir