WHO European Region (EUR) 2012-2013
Currently, there are seven bilateral influenza cooperative agreements that support influenza activity in the European Region. These cooperative agreements are with ministries of health or other institutions that work with the U.S. Centers for Disease Control and Prevention (CDC) to build capacity in order to routinely identify, diagnose and respond to seasonal and pandemic influenza.
Direct Country Support
CDC supports the following countries and/or entities via cooperative agreements:
In addition, CDC supports WHO EURO via a cooperative agreement to provide technical and coordination support to Member States. This cooperative agreement also supports influenza activities in Romania.
Core Activities
The core activities of these bilateral agreements are:
- To build sustainable national capacity for the detection, identification and response to seasonal, avian and novel influenza.
- To develop interagency pandemic preparedness plans.
- To strengthen capacity for integrated laboratory and epidemiologic surveillance for influenza-like illness (ILI) and severe acute respiratory infections (SARI), which includes making routine contributions to WHO’s Global Influenza Surveillance and Response System (GISRS) and implementing International Health Regulations 2005 (IHR).
- To develop and train local rapid response and containment teams.
Influenza Division Contacts
Stacey Spivey-Blackford, MS
Project Officer
Extramural Program
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Email: ifm8@cdc.gov
Mark Thompson, PhD
Health Scientist
International Epidemiology and Research Team
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Email: mthompson2@cdc.gov
WHO Regional Office for Europe (EURO)
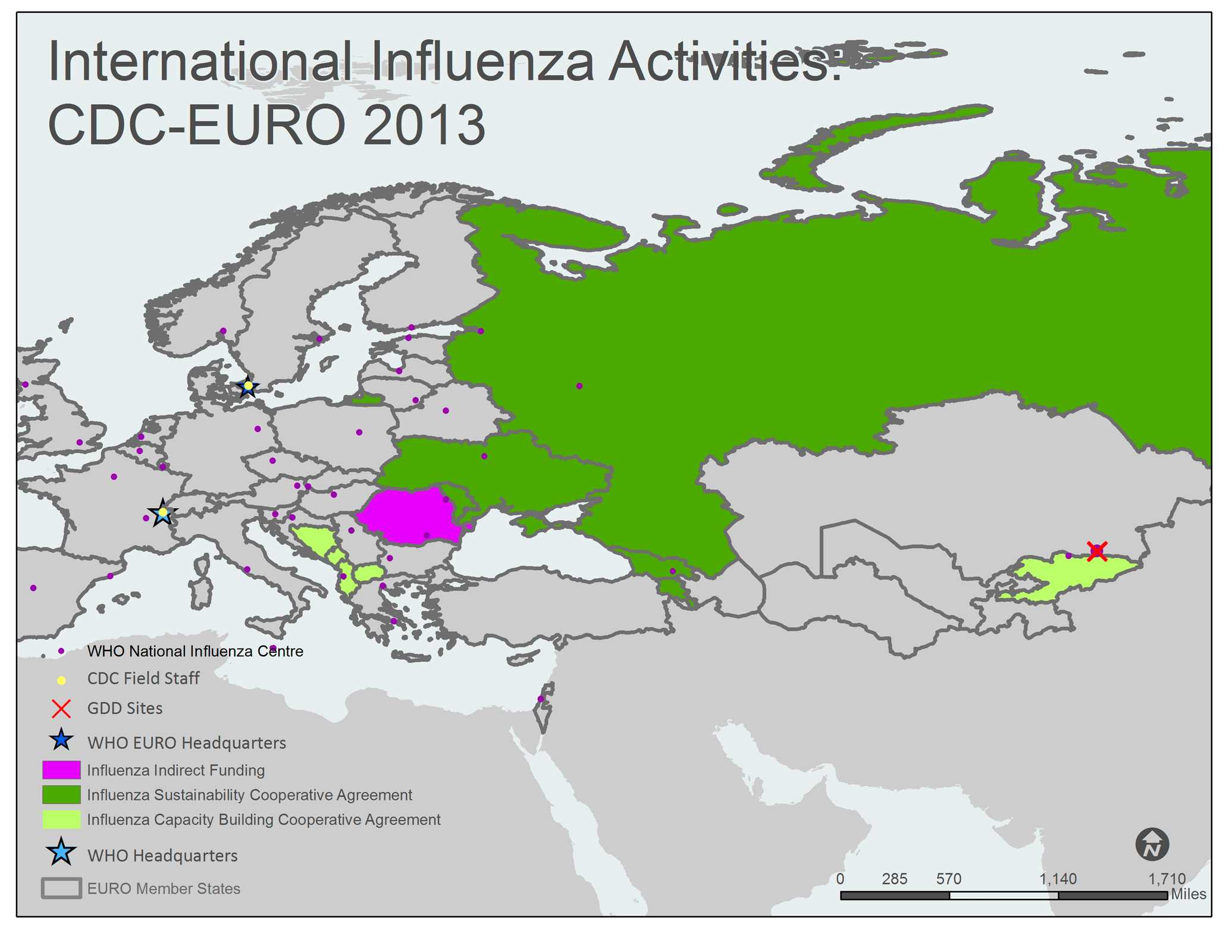
A map of the WHO European Region (EUR) shows all 53 EUR member states/countries. The member countries, outlined with gray borders, include Albania, Andorra, Armenia, Austria, Azerbaijan, Belarus, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Georgia, Germany, Greece, Hungary, Ireland, Israel, Italy, Kazakhstan, Kyrgyzstan, Latvia, Lithuania, Luxembourg, Malta, Monaco, Montenegro, Netherlands, Norway, Poland, Portugal, Republic of Moldova, Romania, Russian Federation, San Marino, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Tajikistan, The Former Yugoslav Republic of Macedonia, Turkey, Turkmenistan, Ukraine, United Kingdom of Great Britain and Northern Ireland, and Uzbekistan.
Countries with yellow shading indicate that the Influenza Division provides project funding and technical assistance through Capacity Building Cooperative Agreements. Kyrgyzstan and the Southeast European Center for Surveillance and Control of Infectious Diseases (SECID) priority countries (Albania, Bosnia and Herzegovina, The Former Yugoslav Republic of Macedonia, and Montenegro) are shaded yellow on the map. Armenia, Georgia, Moldova, the Russian Federation, and Ukraine are shaded green to indicate that they have a Sustainability Cooperative Agreement. Romania is shaded pink to indicate that they receive indirect funding from the Influenza Division.
CDC Influenza Division Field Staff, indicated by a yellow dot, are located at the WHO Regional Office for Europe in Copenhagen, Denmark and at WHO Headquarters in Geneva, Switzerland.
The Global Disease Detection [GDD] Site, indicated by the red “X”, is located in Kazakhstan.
WHO National Influenza Centers (NICs), indicated by a purple dot, are located in Albania (Tirana), Austria (Vienna), Belarus (Minsk), Belgium (Brussels), Bulgaria (Sofia), Croatia (Zagreb), Czech Republic (Prague), Denmark (Copenhagen), Estonia (Tallinn), Finland (Helsinki), France (Lyon and Paris), Georgia (Tbilisi), Germany (Berlin), Greece (Athens and Thessaloniki), Hungary (Budapest), Iceland (Reykjavik), Ireland (Dublin), Israel (Tel Hashomer), Italy (Rome), Kazakhstan (Almaty), Kyrgyzstan (Kyrgyzstan), Latvia (Riga), Lithuania (Vilnius), Luxembourg (Luxembourg), Malta (Msida), Netherlands (Rotterdam), Norway (Oslo), Poland (Warsaw), Portugal (Lisboa), Romania (Bucharest [2] and Lasi), Russia (Moscow and St. Petersburg), Serbia (Belgrade and Novi Sad), Slovakia (Bratislava), Slovenia (Ljubljana), Spain (Barcelona, Madrid and Valladolid), Sweden (Solna), Switzerland (Geneva), Turkey (Ankara), Ukraine (Kiev), and United Kingdom (Aberdeen, London and Glasgow).
The WHO Regional Office for Europe (EURO), indicated by a blue star, is located in Copenhagen, Denmark. WHO Headquarters is located in Geneva, Switzerland.
Highlights
- Published 32 weekly EuroFlu influenza surveillance bulletins; three issues of Flu Focus; seasonal influenza vaccination recommendations; regular Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and avian influenza A(H7N9) summaries; four peer-reviewed articles and one textbook chapter.
- Conducted the first WHO/Europe Flu Awareness Day; one subregional and one regional (jointly with ECDC) influenza surveillance meeting; missions to nine countries to strengthen surveillance, vaccine uptake and conduct burden studies; a multi-country severe acute respiratory infection (SARI) risk factor study and training in data management; facilitation of pandemic workshops in six countries to strengthen response to avian influenza A(H7N9).
- Recognized the National Influenza Centre, Republic of Moldova.
- Participated/presented at 15 international and WHO meetings.
U.S. CDC Direct Support
The second five-year cooperative agreement (CoAg) Surveillance and Response to Pandemic and Avian Influenza by Regional Offices of the World Health Organization (WHO) began in September 2011 and entered its second year in 2012. The WHO Regional Office for Europe (WHO/Europe) in Copenhagen, Denmark, serves 53 Member States (MS) with a population exceeding 900,000 million. Influenza activities are conducted by the Influenza & Other Respiratory Pathogens programme (IRP). In 2012/13, IRP ran the Regional surveillance platform, EuroFlu; organized the European Region influenza surveillance network Annual Meeting with the European Centre for Disease Prevention and Control (ECDC); provided training and technical assistance to Member States to strengthen influenza (outpatient and SARI) surveillance, as well as conducted studies to determine risk factors for severe disease due to influenza and burden of disease; supported the sharing of influenza viruses within GISRS; implemented the second survey on vaccine policies and uptake in the Region and piloted a tool to increase influenza vaccine uptake in health care workers; supported pandemic plan revisions; and developed and disseminated technical guidance related to the outbreaks of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and avian influenza A(H7N9).
In 2013/2014, IRP will continue the above activities and, in addition, will conduct a series of workshops and trainings in clinical management, outbreak response and laboratory detection to prepare countries to respond to cases of avian influenza A(H7N9) or other forms of severe influenza. At least one National Influenza Centre (NIC) will be assessed for recognition by WHO and external quality control for virus isolation: and antiviral susceptibility testing, coupled with follow-up training for participating laboratories, will be conducted. Further improvements to data analysis and presentation in the EuroFlu bulletin will be made. The joint WHO/Europe-ECDC pandemic guidance will be finalized, in-line with the revised WHO global pandemic guidance.
Surveillance
WHO/Europe publishes the EuroFlu bulletin in English and Russian (www.euroflu.org) weekly during the influenza season (weeks 40 to 20) and biweekly outside of the season. Surveillance is coordinated with ECDC. The timing and duration of the 2012/2013 season were similar to previous seasons in the European Region, although influenza activity and morbidity rates (outpatient and SARI) were higher than in the previous season. All three seasonal influenza viruses co-circulated, 63% were influenza A and 37% influenza B; and of the sub-typed influenza A viruses, about two-thirds were A(H1N1)pdm09 and one third A(H3N2). Excess all-cause mortality in up to 18 countries that participate in the EuroMoMo project was only observed in those above age 64 and was comparable with the previous season. Circulating viruses matched with those recommended by WHO for inclusion in the 2012/2013 northern hemisphere seasonal influenza vaccine and there was no indication of increased resistance to neuraminidase inhibitors.
Surveillance Activities
- Summarized and published the EuroFlu bulletin with data from, on average, 47/53 Member States (MS), including sentinel SARI data from 12 MS.
- Published a report describing key features of the 2012/2013 influenza season.
- Conducted the third joint WHO/Europe and ECDC annual influenza meeting for MS and a sub-regional surveillance network meeting for newly independent states.
- Piloted the quantification of the qualitative indicator for influenza activity on the EuroFlu platform to aid interpretation of data and comparison across seasons.
- Performed a multi-country study to identify risk factors for severe outcomes in SARI cases from sentinel SARI surveillance.
- Published WHO/Europe recommendations for seasonal influenza vaccination [57KB, 2 pages], including a summary of WHO recommendations on influenza vaccine composition and target groups for vaccination.
- Collaborated with the Vaccine European New Integrated Collaboration Effort (VENICE) and ECDC on the second regional survey on influenza vaccine policy and uptake.
- Developed and piloted a tool to aid MS in targeting health care workers for influenza vaccination in one country.
- Conducted the first Flu Awareness Day to promote vaccination in the Region and answer any questions about influenza prevention and treatment, through web-articles, a twitter chat and a video “The 5 things everyone needs to know about flu.”
Laboratory
In the European Region, 41 of 50 MS with influenza surveillance now have NICs recognized by WHO. Through the CDC cooperative agreement, NICs in the European Region receive training in influenza laboratory techniques, support to improve laboratory quality, assistance with shipment of viruses to WHO collaborating centres for reference and research on influenza (WHO CC), and reagents for influenza testing.
Laboratory Activities
- The NIC in Republic of Moldova was formally recognized by WHO, bringing the number of countries in the Region with a WHO-recognized NIC to 41.
- A total of 26 countries sent 29 shipments of viruses to the WHO CC before the WHO Consultation on the Composition of Influenza Virus Vaccines for the Northern Hemisphere 2013/2014. The global WHO shipment fund was used to ship 17 of those shipments from 15 countries. A WHO training on shipping infectious substances was provided to 24 specialists from national, regional and sub-regional levels and reference laboratories in Kazakhstan.
- Sixty-one influenza laboratories in 47 MS participated in the WHO EQAP and 78% achieved correct results (10 out of 10)— compared with 67% in 2012.
- Staff from two NICs received training at the WHO CC, National Institute for Medical Research, London, United Kingdom.
Preparedness
In response to lessons learned from the 2009 pandemic, WHO/Europe and ECDC developed joint pandemic preparedness guidance for the Region, to be published in 2014. IRP also provided assistance in the development of WHO pandemic guidance and implementation of the Preparedness Framework. Assistance was provided to countries in developing their pandemic plan.
In response to the MERS-CoV and avian influenza A(H7N9) outbreaks, WHO/Europe provided support to countries, mainly by disseminating key information in regular, timely summaries and posting WHO recommendations and guidance on the WHO/Europe website in English and Russian. Assistance was provided to NICs for development of PCR capacity for the detection of these emerging viruses. Trainings in clinical management of severe influenza were conducted in Azerbaijan and Uzbekistan.
Preparedness Activities
- A workshop on pandemic preparedness held for 23 countries of the South-eastern Europe Health Network, the newly independent states, Israel, Switzerland and Turkey.
- Finland revised and published its national pandemic plan.
- In collaboration with GIZ (Deutsche Gesellschaft für Internationale Zusammenarbeit), support was provided to Tajikistan in revising and implementing its national pandemic plan.
- The WHO/Europe influenza website was upgraded to include information on emerging respiratory viruses, including avian influenza A(H7N9) and MERS-CoV.
- IRP staff co-authored Chapter Nine (Pandemic Preparedness) of the 2nd edition of Pandemic Influenza textbook.
- Approximately 80 clinicians were trained in the clinical management of severe influenza cases in Azerbaijan and Uzbekistan in September 2013.
Training
WHO/Europe, in conjunction with selected Member States, hosted the following regional and inter-country trainings in 2012/2013:
- Staff from the NICs in Russia and Serbia received training at the WHO CC, National Institute for Medical Research, London, UK.
- WHO training on shipping infectious substances was provided to 24 specialists from national, regional and sub-regional levels and reference laboratories in Kazakhstan.
- Training in data analysis and management [124 KB, 7 pages] for seven countries conducting sentinel SARI surveillance and participating in the multi-country study on risk factors for severe disease was conducted in collaboration with CDC and the University of Nijmegen Medical Centre, the Netherlands.
- Training on sentinel surveillance was held for approximately 200 sentinel network clinicians and laboratory specialists in Romania.
- A training in the clinical management of severe acute respiratory infections focused on the current A(H7N9) outbreak was provided in Azerbaijan and Uzbekistan.
Published Papers
The IRP team regularly contributes to peer-reviewed articles for medical journals, with the intention of disseminating information about the work done in collaboration with Member States to a wider audience. The following articles were co-authored by IRP members during 2012–2013:
Palm D, Pereyaslov D, Vaz J, Broberg E, Zeller H, Gross D, et al. “Laboratory capability for molecular detection and confirmation of novel coronavirus in Europe, November 2012”, Euro Surveill. 2012;17(49).
Brown C and Hegermann-Lindencrone M. “Pandemic preparedness” in Van-Tam J and Sellwood C (eds) (2012) Pandemic Influenza, 2nd Edition. CABI, Wallingford, United Kingdom.
Brown C. “The role of the WHO Regional Office for Europe in response to seasonal, avian and pandemic influenza.” Bundesgesundheitblatt, Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz, January 2013, Volume 56, Issue 1, pp 47–55. Abstract.
Jorgensen P, Wasley A, Mereckiene J, Cotter S, Weber JT, Brown C, “Unequal access to vaccines in the WHO European Region during the A(H1N1) influenza pandemic in 2009.” Vaccine; 31 (38) 2013, 4060–62.
Thompson CI, Lackenby A, Daniels RS, McCauley JW, Pereyaslov D, Broberg EK, et al. “Evaluation of influenza virus antiviral susceptibility testing in Europe: results from the first external quality assessment exercise.” J Clin Virol. 2013;56(3):212–8.
Contacts
Caroline Brown, PhD
Programme Manager, Influenza and Other Respiratory Pathogens Programme
WHO Regional Office for Europe
World Health Organization
Copenhagen, Denmark
Email: cbr@euro.who.int
Pernille Jorgensen, MSc, MPH
Epidemiologist, Influenza and Other Respiratory Pathogens Programme
WHO Regional Office for Europe
World Health Organization
Copenhagen, Denmark
Email: pej@euro.who.int
Diane Gross, DVM, PhD
Epidemiologist, Influenza and Other Respiratory Pathogens Programme
WHO Regional Office for Europe
World Health Organization
Copenhagen, Denmark
Email: dgo@euro.who.int
Dmitriy Pereyaslov, MD, MPH
Technical Officer, Laboratory
Influenza and Other Respiratory Pathogens Programme
WHO Regional Office for Europe
World Health Organization
Copenhagen, Denmark
Email: pdm@euro.who.int
Michala Hegermann-Lindencrone, MPH
Technical Officer
Influenza and Other Respiratory Pathogens Programme
WHO Regional Office for Europe
World Health Organization
Copenhagen, Denmark
Email: mhl@euro.who.int
Tamara Meerhoff, PhD
Researcher, Temporary Advisor to WHO Europe
Radboud University Nijmegen Medical Centre
Nijmegen, The Netherlands
Email: T.Meerhoff@elg.umcn.nl
Ganna Bolokhovets, PhD
Consultant
WHO Regional Office for Europe
World Health Organization
Copenhagen, Denmark
Email: gbo@euro.who.int
- Page last reviewed: July 8, 2014
- Page last updated: July 8, 2014
- Content source:
Error processing SSI file


 ShareCompartir
ShareCompartir