WHO Region of the Americas (AMR) 2012-2013
As of FY 2013, there are four bilateral influenza cooperative agreements in the Region of the Americas. These agreements with ministries of health (MOH) or institutions designated by the MOHs work with the Pan American Health Organization (PAHO)/the World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC) to build capacity to routinely identify and respond to seasonal and novel influenza strains across the Americas.
Direct Country Support
CDC direct country support via cooperative agreements is established in the following countries:
In addition, CDC supports PAHO via a cooperative agreement. CDC also supports activities with the Center for Central America and Panama ( CDC-CAP [683 KB, 2 pages] ) at the CDC, Global Disease Detection (GDD) site in Guatemala. These activities support programs in eight Central American/Caribbean countries including Belize, Guatemala, El Salvador, Honduras, Nicaragua, Costa Rica, Panama, and the Dominican Republic.
Core Activities
The core activities of our bilateral agreements and technical assistance are:
- To build sustainable national capacity to identify and respond to seasonal influenza, pandemic influenza and other emerging diseases in accordance with International Health Regulations 2005 (IHR).
- To make routine contributions of surveillance and virology data to WHO’s Global Influenza Surveillance and Response System (GISRS).
- To increase the geographic reach of WHO GISRS.
- To provide earlier access to critical virus isolates from humans and birds for WHO GISRS.
- To increase the numbers of shipments and influenza isolates provided by local influenza labs for analysis by WHO Collaborating Centers (CC).
- To develop sustainable epidemiologic and virologic surveillance systems for severe influenza in order to gain understanding of the disease and economic burden caused by influenza and other respiratory viruses.
- To develop and sustain interagency national preparedness plans.
- To develop and train local rapid response and containment teams.
- To sustain and leverage quality sentinel surveillance and study cohorts to explore the potential cost-effectiveness of expanding vaccination and incorporating new delivery mechanisms, formulations, and novel influenza vaccines in the PAHO Region.
In addition to our bilateral work, we also partner with the U.S. Naval Medical Research Unit No. 6 (NAMRU-6) in Lima, Peru to jointly support South American countries that are starting influenza surveillance.
Influenza Division Contacts
Sara Mirza, PhD, MPH
Epidemiologist
Extramural Program
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Email: smirza@cdc.gov
Eduardo Azziz-Baumgartner, MD, MPH
Medical Officer/Epidemiologist
International Epidemiology and Research Team
Influenza Division, NCIRD
U.S. Centers for Disease Control and Prevention
Email: eha9@cdc.gov
WHO Pan American Health Office (PAHO)
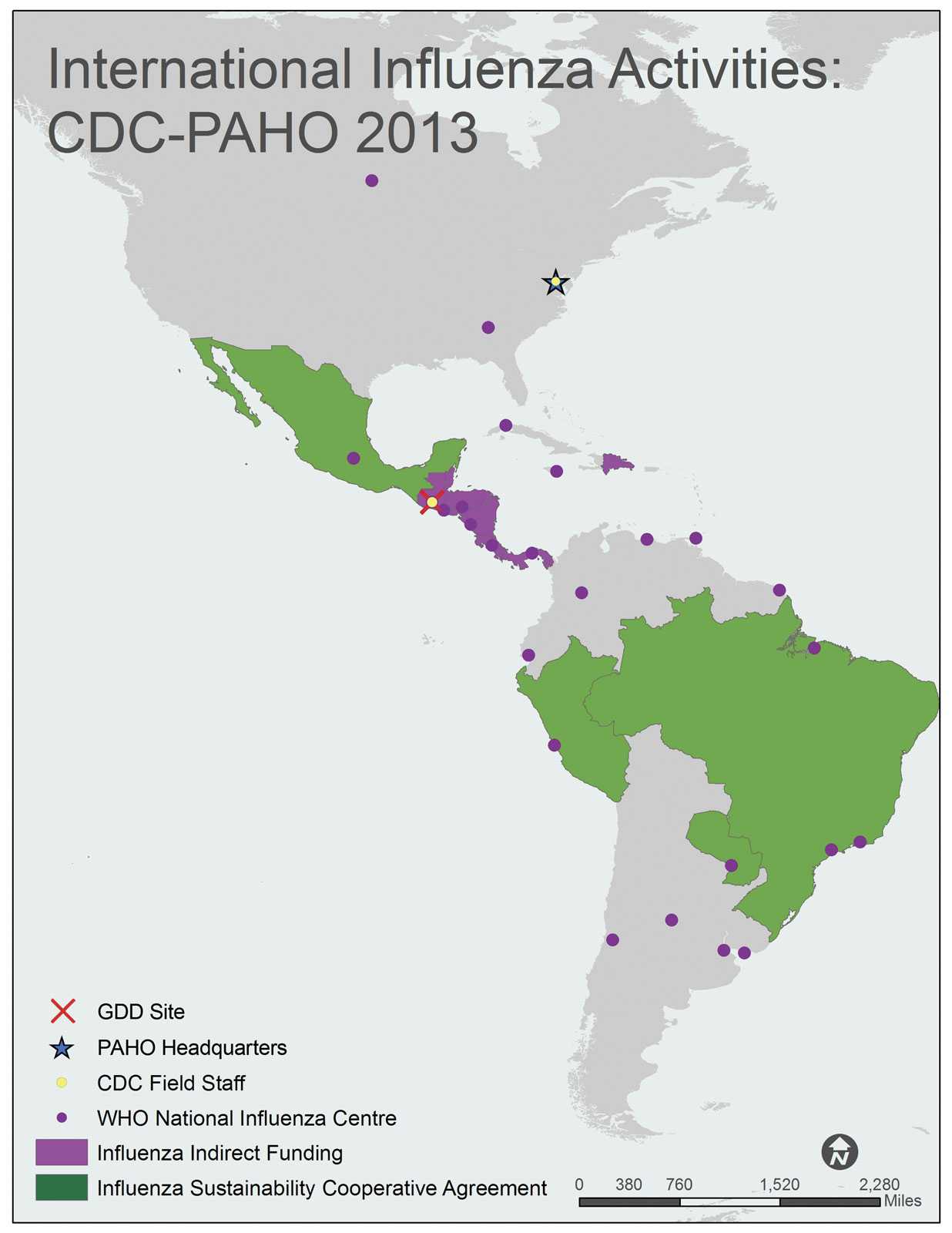
A map of the WHO Region of the Americas (AMR) shows all 35 AMR member states/countries. The member countries, include Antigua and Barbuda, Argentina, Bahamas, Barbados, Belize, Bolivia, Brazil, Canada, Chile, Columbia, Costa Rica, Cuba, Dominica, Dominican Republic, Ecuador, El Salvador, Grenada, Guatemala, Guyana, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Paraguay, Peru, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago, United States of America, Uruguay, and Venezuela.
Countries with shading indicate that the Influenza Division provides project funding and technical assistance through cooperative agreements. Brazil, Mexico, Paraguay and Peru are shaded green to indicate Sustainability Cooperative Agreements. Belize, Costa Rica, Dominican Republic, El Salvador, Guatemala, Honduras, Nicaragua and Panama are shaded pink to indicate that they receive indirect funding from the Division.
CDC Influenza Division Field Staff, indicated by a yellow dot, are located in the following city: Washington, DC.
The Global Disease Detection [GDD] Site, indicated by the red “X”, is located in Guatemala.
WHO National Influenza Centers (NICs), indicated by a purple dot, are located in Argentina (Buenos Aires, Córdoba, and Mar Del Plata), Brazil (Ananindeua, Sao Paulo, and Rio de Janeiro), Canada (Winnipeg), Chile (Santiago), Columbia (Bogota), Costa Rica (Cartago), Cuba (Havana), Ecuador (Guayaquil), El Salvador (San Salvador), France-French Guiana (Cayenne), Guatemala (Barcenus), Honduras (Tegucigalpa), Jamaica (Kingston), Mexico (Mexico City), Nicaragua (Managua), Panama (Panama City), Paraguay (Asunción), Peru (Lima), Trinidad and Tobago (Port of Spain), United States of America (Atlanta, Georgia), Uruguay (Montevideo), and Venezuela (Caracas).
The Pan American Health Organization (PAHO) Headquarters, indicated by a blue star, is located in Washington, DC (USA).
Highlights
Enhancing SARI surveillance
- Technical support to 13 countries in Latin America and the Caribbean (LAC) for the implementation of SARI surveillance (Barbados, Bolivia, Chile, Colombia, Costa Rica, Dominica, Ecuador, Honduras, Jamaica, Paraguay, St. Vincents and the Grenadines, Suriname, and Trinidad and Tobago).
- Thirteen countries (62 hospitals) in LAC provide PAHO weekly SARI surveillance data (laboratory and clinical data).
- Locations of hospitals (number of hospitals): Barbados (1), Bolivia (8), Chile (6), Colombia (7), Costa Rica (7), Dominica (1), Ecuador (16), Honduras (3), Jamaica (6), Paraguay (5), St Vincents and the Grenadines (1), Suriname (1), and Trinidad and Tobago (1).
- Developed an information system, PAHOFlu in Spanish, which integrates SARI laboratory and epidemiologic data and is being used in two LAC countries.
- System will be translated into English this project-year.
- Countries using the information system (number of labs/hospitals): Bolivia (8) and Chile (6).
- Weekly virologic data compiled, analyzed, and disseminated from 27 NICs and national laboratories in the Americas (available at http://ais.paho.org/phip/viz/ed_flu.asp).
- Trained 135 health care workers (HCW) from five LAC countries (Bolivia, Colombia, Costa Rica, Guyana, Honduras) in SARI surveillance; have plans to train an additional 250 HCW from 7 countries (Barbados, Chile, Colombia, Ecuador, Guyana, Paraguay, St Vincent’s and the Grenadines).
- Conducted a meeting bringing together epidemiologists and laboratorians to discuss surveillance for other respiratory virus (ORV) using current influenza platforms.
- PAHO National Influenza Center (NIC) meeting of epidemiologists and laboratorians from LAC will be held.
Strengthening laboratory capacity to detect influenza and other respiratory viruses
- Supported LAC NICs shipment of samples to WHO-CC for characterization.
- Purchased equipment, reagents and supplies for real-time RT-PCR and IFI for 17 NICs and labs.
- Provided training for laboratorians from multiple countries.
- Hosted PAHO NIC meeting of epidemiologists and laboratorians from LAC (September 2013).
- 32 LAC NICs and labs participated in 2012 WHO influenza EQAP.
- 27 countries routinely share virologic influenza and ORV data with PAHO.
- IATA-based WHO training for laboratorians from all LACs NICs (planned).
Develop influenza and ORV disease burden estimates
- Ten-year trend analysis of respiratory disease mortality in LAC submitted for publication.
- Influenza-associated mortality in LAC analysis.
U.S. CDC Direct Support
The five-year cooperative agreement Surveillance and Response to Seasonal and Pandemic Influenza by Regional Offices of the World Health Organization (WHO) began in September 2011 and is in its second year of a five-year cooperative agreement.
The WHO Regional Office for the Americas (AMRO/PAHO) is located in Washington DC, Unites States. The Office serves 35 countries; together their population exceeds 953.6 million people. Member countries include Anguilla, Antigua and Barbuda, Argentina, Aruba, Bahamas, Barbados, Belize, Bermuda, Bolivarian Republic of Venezuela, Bolivia, Brazil, British Virgin Islands, Cayman Islands, Chile, Colombia, Costa Rica, Cuba, Dominica, Dominican Republic, Ecuador, El Salvador, French Guiana, Grenada, Guadeloupe, Guatemala, Guyana, Haiti, Honduras, Jamaica, Martinique, Mexico, Montserrat, Netherland Antilles, Nicaragua, Panama, Paraguay, Peru, Puerto Rico, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago, Turks and Caicos, United States, and Uruguay.
In 2012–2013, PAHO provided training and technical assistance to member countries to support routine influenza SARI surveillance and build capacity to strengthen preparedness, surveillance and response and laboratory capacity. PAHO focused on updating the regional influenza surveillance guidelines based on the new WHO standards, enhancing the capacity to monitor respiratory disease activity (SARI surveillance), promoting lab participation in Global Influenza Surveillance Network (GISN) and implementing national surveillance systems for unusual SARI cases (e.g. MERS-CoV and H7N9).
Surveillance
To address the Region’s need for improved SARI and unusual event surveillance, PAHO drafted the Protocol for Nationwide Enhanced SARI Surveillance. It was implemented in selected Caribbean countries and Uruguay, and is ongoing in Chile, Honduras, and Paraguay; Colombia, Ecuador, and Peru have developed workplans to establish this surveillance and several other countries are also considering implementation. Complimentary to this, PAHO is developing an information system for data entry, hospital-laboratory data linkage, and data output automation; it was implemented in Uruguay and is being modified for use in other countries. Finally, for long-term sustainability, the strategy was adopted to integrate SARI surveillance into systems being developed for health-care associated infections and mandatory reportable disease surveillance. PAHO is working with partners in the Region to develop a five-day training course to sensitize stakeholders and train health-care workers on principals of surveillance, response, outbreak investigation, and structuring hospital departments of epidemiology.
Surveillance Activities
- Technical support to 13 countries in Latin America and the Caribbean (LAC) to support SARI surveillance implementation.
- Thirteen countries (62 hospitals) in LAC providing weekly data about SARI surveillance (laboratory and clinical data) to PAHO.
- Locations of hospitals (number of hospitals): Barbados (1), Bolivia (8), Chile (6), Colombia (7), Costa Rica (7), Dominica (1), Ecuador (16), Honduras (3), Jamaica (6), Paraguay (5), St. Vincents and the Grenadines (1), Suriname (1), and Trinidad and Tobago (1).
- Developed an information system, PAHOFlu in Spanish, which integrates SARI laboratory and epidemiologic data and is being used in two LAC countries.
- System will be translated into English this project-year.
- Countries using the information system (number of labs/hospitals): Bolivia (8) and Chile (6).
- Compiled, analyzed, and disseminated weekly virologic data from 27 NICs and national laboratories in the Americas (available at http://ais.paho.org/phip/viz/ed_flu.asp).
- Trained 135 health care workers (HCW) from five LAC countries in SARI surveillance with plans to train an additional 250 HCW from 7 countries.
- Conducted a meeting bringing together epidemiologists and laboratorians to discuss using influenza platforms for surveillance of other respiratory viruses.
- PAHO National Influenza Center (NIC) meeting will be conducted involving both epidemiologists and laboratorians from LAC.
Laboratory
To address challenges identified during the 2009 influenza pandemic, PAHO directed resources to increase laboratory capacity in the Region for influenza and other respiratory viruses through the purchase of automated extractors and vacuum extractors for processing specimens by real-time RTPCR, limited decentralization of real-time RT-PCR, providing refresher courses for real-time RT-PCR and immunofluorescence, and participating in the WHO External Quality Assessment Project (EQAP). Through a PAHO-CDC collaboration, selected countries (Chile, Panama, Colombia, Caribbean) have piloted diagnostics for ORV. Additionally, to develop laboratorians capacity to analyze and disseminate their data, PAHO collaborated with the University of North Carolina at Chapel Hill to develop a 2.5-day training course on the epidemiologic analyses of influenza laboratory data. More than 45 representatives from PAHO region were trained, and the course is available online in English and Spanish. Through these contributions, the LAC regional laboratory network now consists of 23 National Influenza Centers.
Laboratory Activities
- Supported LAC NIC’s shipment of samples to WHO CC for characterization.
- Purchased equipment, reagents and supplies for real-time RT-PCR and IFI for 17 NICs and labs (Bolivia, Chile, Colombia, Costa Rica, Dominican Republic, Ecuador, El Salvador, Honduras, Guatemala, Jamaica, Nicaragua, Panama, Paraguay, Peru, Suriname, Uruguay, Venezuela, CARPHA).
- Planned training of laboratorians from
- Colombia and Dominican Republic in molecular diagnosis of influenza at the national laboratory in Chile (one-week).
- Bolivia and Ecuador in viral isolation for influenza at Gorgas Institute in Panama (two weeks).
- Brazil and Chile in serologic methodologies at CDC (two-weeks).
- Argentina, Brazil, Chile, and Mexico in molecular diagnosis of ORV at Adolfo Lutz Institute in Brazil (CDC-PAHO one week course, completed).
- IATA-based WHO training for laboratorians from all LACs NICs.
- PAHO NIC meeting (September 2013) involving epidemiologists and laboratorians from LAC.
- Twenty-seven countries (Argentina, Bolivia, CARPHA (Anguilla, Barbados, Belize, Bermuda, Cayman Islands, Dominica, St. Lucia, St. Vincent & the Grenadines, Suriname, Trinidad & Tobago), Chile, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, El Salvador, Guatemala, Honduras, Jamaica, Mexico, Nicaragua, Panama, Paraguay and Peru) routine share their influenza and ORV virologic data with PAHO.
Preparedness
PAHO has been working with countries, in this current WHO Pandemic Alert phase for Avian Influenza, to review their Surveillance for Severe Acute Respiratory Illnesses. A particular area of focus is strengthening the role of the laboratory in the reporting and feedback of the SARI surveillance system.
In light of current health risks of viruses with pandemic potential (e.g. MERS-CoV and H7N9), PAHO conducted a meeting with subject matter experts from CDC, WHO, country influenza coordinators to update a regional guidance and refine unusual SARI event surveillance guidelines. Recommendations of this meeting were incorporated into the development of an unusual SARI event surveillance training course and pilot. These are being incorporated into a guidance document and subsequent trainings will be provided to the Member Countries.
Preparedness Activities
- Belize - The Ministry of Health (MOH) reviewed its SARI surveillance system (July 2013) to identify areas for strengthening. In response to the WHO declaration of ALERT for Pandemic Influenza following reports of Avian Influenza in Asia, reporting sites were asked to increase sampling of SARI cases from 1/5 to all cases. Laboratory management issues regarding quality and reporting of influenza have been identified and are being followed up with PAHO and CARPHA support.
- Barbados - The MOH conducted an assessment of its SARI surveillance system (August 2013), focusing on the laboratory component. The MOH also conducted a two hour update for staff at the Queen Elizabeth Hospital on pandemic influenza plans, avian influenza and novel coronavirus, SARI surveillance in Barbados, and a skills-building training on nasopharyngeal sampling. PAHO will follow up with training on PCR techniques and quality assurance methods for the public health laboratory.
- Guyana - The MOH requested PAHO support to review its National Influenza Pandemic Plan following the issuance of the new WHO Pandemic Influenza Guidance.
- Suriname - The MOH requested PAHO support to expand its SARI surveillance sites to include two hospitals in the capital, Paramaibo and to review its national laboratory system for respiratory virus testing.
Training
- Completed four country missions; 12 more are planned. Visits include monitoring and evaluating the SARI surveillance sites and laboratories, conducting SARI surveillance trainings, and developing workplans.
- Trained 135 local level health care workers from five countries in SARI surveillance. Additional training for 250 health care workers from seven countries is planned.
- Participated in laboratory training to strengthen capacity to diagnose influenza and ORV.
- Participated in the 2012 WHO EQAP for influenza (22 countries from LAC).
- Conducted a viral isolation training course with two countries in Panama (May 2013).
- Participated in a joint PAHO-CDC training in Brazil for strengthening laboratory capacity and epidemiologic data integration in ORV surveillance. These countries evaluated PCR kits using a CDC proficiency panel and a retrospective analysis re-testing SARI cases from 2012 using ORV PCR to assess IFA concordance and epidemiologic features of different viruses.
Contacts
Rakhee Palekar, MD, MPH
Advisor, Viral Diseases
Communicable Diseases and Health Analysis
Pan American Health Organization
Washington, DC
Email: palekarr@paho.org
Mauricio Cerpa, MD, MPH
Influenza Surveillance Specialist
Communicable Diseases and Health Analysis
Pan American Health Organization
Washington, DC
Email: cerpamau@paho.org
Jairo Mendez, PhD
Influenza Laboratory Specialist
Communicable Diseases and Health Analysis
Pan American Health Organization
Washington, DC
Email: ricoj@paho.org
Tiffany D’Mello, MPH, MBA
Influenza Surveillance Specialist
Communicable Diseases and Health Analysis
Pan American Health Organization
Washington, DC
Email: dmellot@paho.org
Robert Lee, MD, MPH
Advisor, Policy Analysis and Program Planning
Communicable Diseases and Health Analysis
Pan American Health Organization
Washington, DC
Email: leerober@paho.org
Oöna Bilbao, MSc
Project Support Specialist
Communicable Diseases and Health Analysis
Pan American Health Organization
Washington, DC
Email: bilbaoo@paho.org
- Page last reviewed: July 8, 2014
- Page last updated: July 8, 2014
- Content source:
Error processing SSI file


 ShareCompartir
ShareCompartir