National Early-Season Flu Vaccination Coverage, United States, November 2016
Figure 1.
Influenza (flu) is a contagious respiratory illness that can be serious, sometimes resulting in hospitalization and death. Anyone can get sick from the flu, but some people, including older people, young children, people with certain health conditions, and pregnant women, are at higher risk for flu-related complications such as pneumonia.
Flu vaccination is the best way to prevent the flu and potentially serious flu-related complications (1, 2). The Centers for Disease Control and Prevention (CDC) recommends that everyone 6 months and older get a flu vaccination each flu season (3).
This report summarizes data from the National Immunization Survey-Flu (NIS-Flu) for children 6 months through 17 years and the National Internet Flu Survey (NIFS) for adults 18 years and older residing in the United States. NIS-Flu data were collected by telephone surveys of parents conducted during October 1–November 12, 2016, while the NIFS data were collected through an Internet panel survey conducted during October 27–November 9, 2016. This report provides early flu season estimates of how many people (children and adults) in the United States had received a flu vaccination. Final 2016–17 flu season vaccination coverage estimates will be available at CDC FluVaxView in September 2017.
Key Findings
- Only approximately two of every five children and adults in the United States were vaccinated by early November 2016:
- 39.8% of all persons 6 months and older
- 37.3% of children 6 months through 17 years
- 40.6% of adults 18 years and older
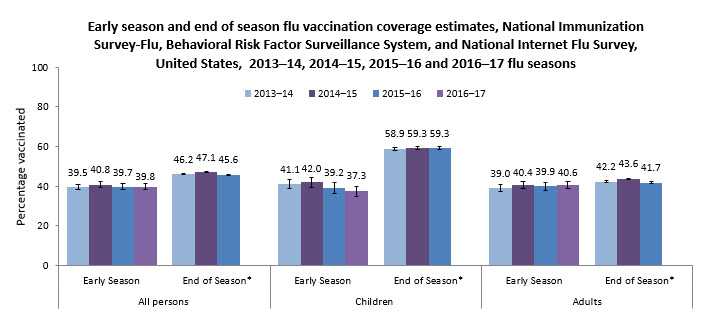
- Early 2016–17 flu season vaccination coverage was similar to coverage at the same time last flu season for children, adults, and all persons 6 months and older.
- Among both children and adults, early-season flu vaccination coverage showed no racial/ethnic differences.
- Among both adults and children, the most common places reported for receiving flu vaccination were medical locations (children: 85.4%, adults: 51.9%). Retail settings (24.3%) and workplaces (17.6%) were other important venues for adults.
Conclusions/Recommendations:
- As of early November 2016, only about 2 out of every 5 persons 6 months and older in the United States had received a flu vaccination.
- Unvaccinated persons are at higher risk of flu illness themselves and of transmitting flu to others, some of whom may be at high-risk of severe illness from flu due to their age (children younger than 5 years, but especially younger than 2 years, and adults 65 years and older) or certain underlying medical conditions.
- People not yet vaccinated this season should get a flu vaccination as soon as possible.
- The Advisory Committee on Immunization Practices (ACIP) recommends the flu shot (inactivated influenza vaccine or IIV) during 2016–17. The nasal spray flu vaccine (live attenuated influenza vaccine or LAIV) should not be used for the 2016–17 season. Parents who previously got their child vaccinated with the nasal spray vaccine should get their child vaccinated with an injectable vaccine this season rather than having their child stay unprotected this flu season (3).
- A provider recommendation to get a flu vaccination is an important factor in a patient’s decision to get vaccinated. Vaccination providers and immunization programs should ensure patients receive recommendations for flu vaccination and expand access to vaccination services.
- Work sites can collaborate with vaccination providers and immunization programs to promote flu vaccination and even offer vaccination at work.
- All providers should routinely assess the flu vaccination status of their patients at every clinical encounter, strongly recommend, and offer flu vaccination.
- Providers who do not stock flu vaccine should refer their patients to a provider who offers flu vaccination and confirm that patients received a flu vaccination (4).
- Standing orders and provider reminders are systems that can prove useful to encourage vaccination in health care settings.
Who Was Vaccinated?
Flu Vaccination Coverage by Age Group
- Flu vaccination coverage as of early November 2016 was 39.8%, similar to coverage at the same time last flu season (39.7%) (Table 1).
Table 1.
Flu vaccination coverage by age group, National Immunization Survey-Flu and National Internet Flu Survey, United States, early 2015–16 and 2016–17 flu seasons |
|||
|---|---|---|---|
|
Age Group |
November 2015 |
November 2016 |
Difference from |
|
Overall (≥ 6 months) |
39.7 ± 1.6 |
39.8 ± 1.5 |
0.1 ± 2.2 |
| Children (6 months–17 years) |
39.2 ± 2.7 |
37.3 ± 2.5 |
-1.9 ± 3.7 |
| Adults (≥ 18 years) |
39.9 ± 1.9 |
40.6 ± 1.7 |
0.7 ± 2.5 |
- Among children 6 months through 17 years, early-season flu vaccination coverage with one or more doses was 37.3% as of early November, similar to coverage at the same time last flu season (Table 2).
- Children in all age groups had similar coverage compared with the same time last season.
- Again, flu vaccination coverage among children decreased as age increased.
- Flu vaccination coverage was highest among children 6 months–4 years (45.0%) and lowest among children 13–17 years (28.7%).
Table 2.
Flu vaccination coverage among children by age group, National Immunization Survey-Flu, United States, early 2015–16 and 2016–17 flu seasons |
|||
|
Age Group |
November 2015 |
November 2016 |
Difference from |
|---|---|---|---|
| All children (6 months-17 years) |
39.2 ± 2.7 |
37.3 ± 2.5 |
-1.9 ± 3.7 |
| 6 months-4 years |
51.7 ± 6.1 |
45.0 ± 4.2 |
-6.7 ± 7.4 |
| 5-12 years |
40.7 ± 3.6 |
39.0 ± 3.8 |
-1.7 ± 5.2 |
| 13-17 years |
26.6 ± 4.5 |
28.7 ± 4.5 |
2.1 ± 6.4 |
- Among adults 18 years and older, flu vaccination coverage as of early November was 40.6%, similar to the same time last flu season (Table 3).
- Adults in all age groups had similar coverage compared with the same time last season.
- Flu vaccination coverage among adults increased as age increased.
- Vaccination coverage among adults was highest among adults ≥65 years (56.6%) and lowest among adults 18–49 years (34.3%).
- Adults 18–64 years with high-risk medical conditions had higher coverage (43.5%) than adults 18–64 years without high-risk conditions (34.0%).
Table 3.
Flu vaccination coverage among adults by age group, National Internet Flu Survey, United States, early 2015–16 and 2016–17 flu seasons |
|||
|
Age Group |
November 2015 |
November 2016 |
Difference from |
|---|---|---|---|
| All adults (≥ 18 years) |
39.9 ± 1.9 |
40.6 ± 1.7 |
0.7 ± 2.5 |
| 18-49 years |
31.8 ± 2.8 |
34.3 ± 2.7 |
2.5 ± 3.9 |
| 50-64 years |
41.3 ± 3.4 |
41.7 ± 2.9 |
0.4 ± 4.5 |
| 18-64 years |
34.9 ± 2.2 |
36.7 ± 2.0 |
1.8 ± 3.0 |
| 18-64 years with high risk conditions§ |
40.8 ± 4.3 |
43.5 ± 3.8 |
2.7 ± 5.7 |
| 18-64 years without high risk conditions |
32.7 ± 2.6 |
34.0 ± 2.4 |
1.3 ± 3.5 |
| ≥ 65 years |
60.4 ± 3.9 |
56.6 ± 2.7 |
-3.8 ± 4.7 |
Flu Vaccination Coverage by Race/Ethnicity
- For all racial/ethnic groups, coverage among children was similar compared with coverage at the same time last season (Table 4).
- There were no racial/ethnic group differences in flu vaccination coverage among children based on early-season estimates.
Table 4.
Flu vaccination coverage among children by race and ethnicity,|| National Immunization Survey-Flu, United States, early 2015–16 and 2016–17 flu seasons |
|||
|
Racial/Ethnic Group |
November 2015 |
November 2016 |
Difference from |
|---|---|---|---|
| Children (6 months-17 years) |
39.2 ± 2.7 |
37.3 ± 2.5 |
-1.9 ± 3.7 |
| Hispanic |
42.1 ± 6.7 |
39.9 ± 6.3 |
-2.2 ± 9.2 |
| Non-Hispanic, white only |
35.2 ± 3.1 |
36.0 ± 3.2 |
0.8 ± 4.5 |
| Non-Hispanic, black only |
42.6 ± 6.7 |
36.8 ± 5.8 |
-5.8 ± 8.9 |
| Non-Hispanic, other/multiple races |
48.8 ± 10.4 |
40.1 ± 5.6 |
-8.7 ± 11.8 |
- For all racial/ethnic groups, coverage among adults was similar compared with the same time last season (Table 5).
- There were no racial/ethnic group differences in flu vaccination coverage among adults based on early-season estimates.
Table 5.
Flu vaccination coverage among adults by race and ethnicity,|| National Internet Flu Survey, United States, early 2015–16 and 2016–17 flu seasons |
|||
|
Racial/Ethnic Group |
November 2015 |
November 2016 |
Difference from November |
|---|---|---|---|
| Adults (≥ 18 years) |
39.9 ± 1.9 |
40.6 ± 1.7 |
0.7 ± 2.5 |
| Hispanic |
36.8 ± 5.9 |
43.5 ± 4.5 |
6.7 ± 7.4 |
| Non-Hispanic, white only |
41.6 ± 2.3 |
39.7 ± 2.2 |
-1.9 ± 3.2 |
| Non-Hispanic, black only |
35.5 ± 5.3 |
40.6 ± 4.4 |
5.1 ± 6.9 |
| Non-Hispanic, other/multiple races |
37.9 ± 6.5 |
43.1 ± 5.5 |
5.2 ± 8.5 |
Place of Vaccination
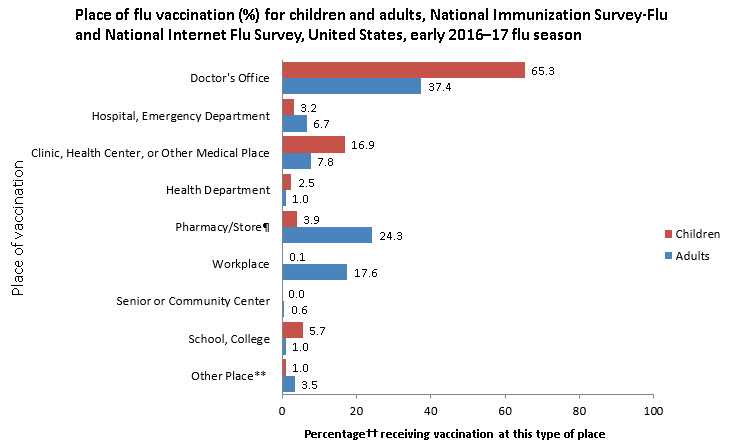
- The most common place of vaccination among both adults and children as of early November 2016 was a doctor’s office (children: 65.3%; adults: 37.4%). Other medical settings for flu vaccination included hospitals or emergency departments (children: 3.2%; adults 6.7%) or clinics, health centers, or other medical places (children: 16.9%; adults: 7.8%) (Figure 2).
- Among children, 5.7% received flu vaccination at school and 3.9% at a pharmacy.
- Other common places of flu vaccination reported by adults included pharmacies (24.3%) and workplaces (17.6%).
- For children, these results are similar to early estimates from the 2015–16 season.
- Early estimates for 2016 indicate that a higher percentage of adults were vaccinated in a doctor’s office (37.4%) compared with last season (33.2%).
Figure 2.
What Can Be Done? (Recommendations)
Approximately 3 out of every 5 persons aged 6 months and older in the United States had not received a flu vaccination by early November. Continued efforts are needed to increase the percentage of the population vaccinated during the next few months in order to reduce the burden of flu, including:
Targeted efforts to increase vaccination coverage among people with high-risk conditions:
- The ACIP has recommended flu vaccination for certain age groups of people and people with certain health conditions because these factors increase the risk for complications from flu infection. Continued emphasis should be placed on vaccinating people at high risk of serious flu complications (e.g., pregnant women, people with chronic pulmonary disease including asthma, chronic cardiovascular diseases, diabetes) (3, 5). Despite the long-standing ACIP recommendations, vaccination levels among persons with medical conditions that increase risk for complications from flu have remained suboptimal (6-8). Therefore, continued efforts to increase vaccination coverage among people with high-risk conditions are necessary. Activities may include:
- Standing orders can reduce the number of missed opportunities for vaccinating persons in multiple settings including clinics, hospitals, pharmacies, and long-term care settings and can increase coverage (9).
- Primary care providers, subspecialists, and pharmacists should routinely assess, recommend, and offer vaccinations when patients access the medical system (4).
- If a provider cannot administer flu vaccination, they should refer their patients to a provider who offers flu vaccination.
Implementation of proven strategies to increase vaccination coverage:
- Increased effort is needed to implement strategies proven to increase flu vaccination coverage, including the following:
- Health care providers should routinely assess, strongly recommend, and offer flu vaccination. Patients are much more likely to get vaccinated when health care providers give a strong recommendation for vaccination coupled with an offer of vaccination (10).
- The National Vaccine Advisory Committee published the revised adult immunization standards (4) to be used by all providers to ensure they assess patients for flu vaccination status at every visit, strongly recommend a flu vaccination if needed, administer the vaccine or refer the patient for vaccination, and document the vaccination in patient’s medical records and an immunization information system.
- Health care providers can increase vaccination rates by using evidence-based strategies such as immunization information systems, provider assessment and feedback, provider reminders, and standing orders, as well combinations of these interventions.
- Health care providers can increase vaccination rates by using evidence-based strategies such as immunization information systems, provider assessment and feedback, provider reminders, and standing orders, as well combinations of these interventions.
- Providers should refer to The Guide to Community Preventive Services which provides guidance on effective interventions for increasing vaccination rates.
- Health care providers should routinely assess, strongly recommend, and offer flu vaccination. Patients are much more likely to get vaccinated when health care providers give a strong recommendation for vaccination coupled with an offer of vaccination (10).
Flu vaccination coverage estimates will continue to be monitored. Early flu vaccination coverage estimates were similar for the 2014–15, 2015–16, and 2016–17 seasons; however, the end-of-season estimates for the 2015–16 flu season showed a 3.4 percentage point decrease in vaccination coverage among people 50–64 years and 3.3 percentage point decrease among those 65 years and older compared with the 2014–15 season. Final 2016–17 flu season vaccination coverage estimates will be examined to determine whether declines continue in these age groups.
Data Sources and Methods
The 2016–17 flu vaccination coverage estimates and findings reported here are early-season estimates. These results will differ from final end-of-season coverage estimates expected in September 2017. End-of-season estimates in Figure 1 are from the NIS-Flu for children and the Behavioral Risk Factor Surveillance System (BRFSS) for adults (CDC – Flu Vaccination Coverage, United States, 2015–16 Influenza Season, Flu Vaccination Coverage, United States, 2014–15 Influenza Season, and Flu Vaccination Coverage, United States, 2013–14 Influenza Season).
The 2016–17 early-season estimates in this report are based on two different data sources. Estimates for children are based on data from the NIS-Flu, while estimates for adults are based on data from the NIFS. NIS-Flu data from October 1 through November 12, 2016, were compared to NIS-Flu data from October 1 through November 14, 2015. NIFS data from October 27 through November 9, 2016, were compared to NIFS data from October 29 through November 11, 2015 (National Early Season Flu Vaccination Coverage, United States, November 2015).
National Immunization Survey-Flu (NIS-Flu)
The NIS-Flu is an ongoing, national list-assisted random-digit-dialed dual-frame landline and cellular telephone survey of households with children. It includes three components: the NIS for children 19–35 months, the NIS-Teen for children 13–17 years, and a short flu module for all other children 6–18 months and 3–12 years not eligible for the NIS and NIS-Teen identified during the household screening process. Respondents 18 years and older were asked if their child had received a flu vaccination since July 1, 2016, and, if so, in which month and year. The survey interviewers conducted the survey in both English and Spanish; interviews conducted in other languages used language-line interpretation services.
Flu vaccination coverage estimates presented in this report are based on interviews conducted from October 1 through November 12, 2016, to cover vaccinations received from July–November, 2016. A total of 24,528 NIS-Flu interviews were completed for children 6 months–17 years. Of these, 4,446 were by landline telephone and 20,082 were by cellular telephone. For reporting place of vaccination, three weeks of NIS-Flu interviews (October 23–November 12, 2016) were combined; the place of vaccination estimates are based on 3,972 vaccinated children.
Flu vaccination coverage estimates represent the approximate cumulative proportion of persons vaccinated as of November 12, 2016. Coverage was calculated using an enhanced estimation strategy that resembles the Kaplan-Meier estimation procedure (11). The flu vaccination coverage estimates represent receipt of at least one dose of flu vaccine. Place of vaccination was estimated using simple weighted proportions. All estimates were weighted based on the probability of selection of the telephone number, including adjustments for nonresponse at the telephone number resolution and household screening stages, probability of selecting the child of interest within the household, and for person nonresponse. The data were also weighted using a ratio adjustment to population controls (age, sex, race/ethnicity, and geographic area). All NIS-Flu estimates reported here were calculated by NORC at the University of Chicago utilizing weights they developed.
National Internet Flu Survey (NIFS)
The adult estimates are based on data from the 2016 NIFS, which was conducted by RTI International and GfK Custom Research, LLC, and sponsored by CDC, to rapidly collect flu vaccination-related data early in the 2016–17 flu season. The survey was conducted using a probability-based Internet panel designed to be representative of the noninstitutionalized U.S. population 18 years and older. The Internet panel survey was conducted in English only.
The sample was stratified by age group and by race/ethnicity. For this ongoing panel, participants are initially chosen by a random selection of residential addresses. Persons in selected households are then invited to participate in the web-enabled KnowledgePanel®. For those who agree to participate but do not already have Internet access, GfK provides both a laptop and Internet access at no cost. People who already have computers and Internet service participate using their own equipment. Panelists receive unique log-in information for accessing surveys online and are sent e-mails throughout each month inviting them to participate in a variety of surveys. The 2016 NIFS sampling design was a single-stage stratified sample with oversampling of select subgroups of particular analytical interest. Twelve mutually exclusive design strata were defined as the interaction of two categorical variables known for all members of the probability-based Internet panel—age (18–49 years, 50–64 years, and 65 years and older) and race/ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, and non-Hispanic, other/multiple races). Independent random samples were selected within each design stratum.
The field period of data collection for the NIFS was October 27–November 9, 2016. A survey invitation was sent to a sample consisting of 7,014 panel members. A total of 4,305 completed the NIFS. All NIFS estimates reported here were calculated by RTI utilizing analysis weights developed by GfK, which adjusted the base weights for survey nonresponse and for coverage of the target population. For this purpose, an iterative proportional fitting (raking) procedure was used to produce final NIFS analysis weights that aligned with respect to all study benchmark marginal distributions simultaneously. The geodemographic dimensions used in the raking procedure included gender, age group, race/ethnicity, education, census region, household income, home ownership status, and metropolitan area. The corresponding population totals for the geodemographic dimensions were obtained from the Current Population Survey (March 2016 Supplement) general-population benchmarks for ages 18 and older.
The raked weights were examined for outliers; a small number of outlier weights were trimmed at the extreme upper and lower tails of the weight distribution. In the final step, the trimmed weights were scaled to the sum of the total sample size of all eligible respondents.
Flu vaccination coverage estimates represent the approximate cumulative proportion of adults vaccinated as of November 9, 2016. The survey responses “don’t know” and “refused” were excluded from the analyses.
Additional Methods
Differences between groups and between the 2015–16 and 2016–17 seasons were determined using t-tests with significance at p<0.05 and assuming large degrees of freedom (thus, using the value of 1.96 for the critical value). Any differences noted as increases or decreases were statistically significant differences; when it is stated that estimates were similar or there was no difference, this indicates that any differences were not statistically significant.
To produce a national estimate of flu vaccination coverage for all persons 6 months and older, the estimates from the NIS-Flu for children and from the NIFS for adults were combined by weighting them by population size (based on census population counts).
Limitations
- The findings reported here are early-season estimates and final end-of-season coverage estimates will likely increase.
- Children 6 months–8 years may require two doses of flu vaccine to optimize immunity (3); estimates in this report reflect parental report of at least one dose—not whether those children requiring two doses were fully immunized.
- NIS-Flu is a telephone survey that excludes households with no cellular or landline telephone service. Noncoverage and nonresponse bias may remain after weighting adjustments.
- The adult estimates in this report are based on the NIFS, an Internet panel survey. Although the Internet panel was probability-based, the estimates may not represent all adults in the United States, and bias may remain after the weighting adjustments.
- All data rely on self-report and are not validated with medical records; validity studies have shown that parental report (for children) and self-report (for adults) may overestimate flu vaccination coverage (12-14).
Authors:
Anup Srivastav,1 Walter W. Williams,2 Tammy A. Santibanez,2 Katherine E. Kahn,1 Yusheng Zhai,1 Peng-Jun Lu,2 Carolyn B. Bridges,2 Amy Parker Fiebelkorn,2 Ashley Amaya,3 Jill A. Dever,3 Marshica S. Kurtz,3 Jessica Roycroft,3 Michael S.S. Lawrence,4 Mansour Fahimi,4 Lin Liu5
1Leidos Inc.
2Immunization Services Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention
3RTI International
4GfK Customs Research
5NORC at University of Chicago
Related Links
- FluVaxView — Influenza Vaccination Coverage
- Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2016–17 Influenza Season
- Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2015–16 Influenza Season
- Standards for Adult Immunization Practice
- Flu Vaccination Coverage, United States, 2015–16 Influenza Season
- National Early Season Flu Vaccination Coverage, United States, November 2015
- Flu Vaccination Coverage, United States, 2014–15 Influenza Season
- National Early Season Flu Vaccination Coverage, United States, November 2014
- Flu Vaccination Coverage, United States, 2013–14 Influenza Season
- National Early Season Flu Vaccination Coverage, United States, November 2013
- Flu Vaccination Coverage, United States, 2012–13 Influenza Season
- National Early Season Flu Vaccination Coverage, United States, 2012–13 Flu Season
- Estimated Influenza Illnesses and Hospitalizations Averted by Vaccination – United States, 2014–15 Influenza Season
- Estimated Influenza Illnesses and Hospitalizations Averted by Vaccination – United States, 2013–14 Influenza Season
- Estimated Influenza Illnesses and Hospitalizations Averted by Influenza Vaccination – United States, 2012–13 Influenza Season
- Flu Vaccination Coverage, United States, 2011–12 Influenza Season
- Flu Vaccination Coverage, National Flu Survey, March 2012
- Results from the November 2011 National Flu Survey – United States, 2011–12 Influenza Season
- National Immunization Surveys
- CDC Influenza Awareness Campaign (media relations toolkit) [506 KB, 19 pages]
- Follow CDC Flu on Twitter: @CDCFlu
References
- CDC. Estimates of Deaths Associated with Seasonal Influenza --- United States, 1976--2007. MMWR Morb Mortal Wkly Rep 2010;59(33):1057-1062.
- CDC. Estimated Influenza Illnesses and Hospitalizations Averted by Vaccination – United States, 2014-15 Influenza Season. Available at: http://www.cdc.gov/flu/about/disease/2014-15.htm.
- Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices – United States, 2016-17 Influenza Season. MMWR Recomm Rep 2016;65(5):1-54.
- National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory Committee: Standards for Adult Immunization Practice. Public Health Rep 2014;129:115-123.
- CDC. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices, 2010. MMWR Recomm Rep 2010;59(RR08):1-462.
- CDC. Flu Vaccination Coverage, United States, 2015-16 Influenza Season. Available at: http://www.cdc.gov/flu/fluvaxview/coverage-1516estimates.htm.
- Lu PJ, O’Halloran A, Ding H, Srivastav A, Williams WW. Uptake of Influenza Vaccination and Missed Opportunities Among Adults with High-Risk Conditions, United States 2013. Am J Med 2016;129(6):636.e1-636.e11.
- Santibanez TA, Lu PJ, O’Halloran A, Meghani A, Grabowsky M, Singleton JA. Trends in Childhood Influenza Vaccination Coverage -- U.S., 2004-2012. Public Health Rep 2014;129(5):417-427.
- Guide to Community Preventive Services. Increasing Appropriate Vaccination: Health Care System-Based Interventions Implemented in Combination. Available at: https://www.thecommunityguide.org/sites/default/files/Vaccination-Health-Care-System-Based-Interventions-Implemented-in-Combination-Archive.pdf [611 KB, 6 pages].
- CDC. Influenza Vaccination Coverage Among Pregnant Women – 2011-12 Influenza Season, United States. MMWR Morb Mortal Wkly Rep 2012;61(38):758-763.
- Ganesh N, Copeland KR, Davis ND, Singleton JA, Santibanez TA. Modeling H1N1 Vaccination Rates. Proc JSM Section on Survey Research Methods 2010:5263-5277.
- Brown C, Clayton-Boswell H, Chaves SS, Prill MM, Iwane MK, Szilagyi PG, et al. Validity of Parental Report of Influenza Vaccination in Young Children Seeking Medical Care. Vaccine 2011;29(51):9488-9492.
- Rolnick SJ, Parker ED, Nordin JD, Hedblom BD, Wei F, Kerby T, et al. Self-Report Compared to Electronic Medical Record Across Eight Adult Vaccines: Do Results Vary by Demographic Factors? Vaccine 2013;31(37):3928-3935.
- Mangtani P, Shah A, Roberts JA. Validation of Influenza and Pneumococcal Vaccine Status in Adults Based on Self-Report. Epidemiol Infect 2007;135(1):139-143.
Footnotes
*End-of-season estimates are from the National Immunization Survey-Flu for children (6 months through 17 years) and the Behavioral Risk Factor Surveillance System for adults (18 years and older) (Flu Vaccination Coverage, United States, 2015–16 Influenza Season, Flu Vaccination Coverage, United States, 2014–15 Influenza Season, and Flu Vaccination Coverage, United States, 2013–14 Influenza Season). The 2016–17 end-of-season estimates will not be available until September 2017.
†All percentages in the table are weighted to the U.S. population.
‡CI=Confidence interval half-width.
§Adults were considered as having a high-risk medical condition if they had ever been told by a doctor or other health professional that they had chronic asthma, a lung condition other than asthma, diabetes, heart disease (other than high blood pressure, heart murmur, or mitral valve prolapse), a kidney condition, a liver condition, obesity, sickle cell anemia or other anemia, a neurologic or neuromuscular condition that makes it difficult to cough, or a weakened immune system caused by chronic illness or by medicines such as chemotherapy, steroids, and transplant medicines taken for chronic illness such as cancer and HIV/AIDS.
||Race-ethnicity is either reported by parent/guardian (NIS-Flu) or self-reported (NIFS). Persons of Hispanic ethnicity may be of any race. “Non-Hispanic, other/multiple races” includes Asians, American Indians or Alaska Natives, Native Hawaiians or other Pacific Islanders, and persons who selected “other” race or multiple races.
¶“Pharmacy/Store” includes pharmacies or drugstores and local supermarkets or grocery stores.
**“Other place” includes military-related places, other schools such as trade schools, residences, and other unspecified nonmedical places.
††Percentage may not add to 100 due to rounding.
- Page last reviewed: December 9, 2016
- Page last updated: December 9, 2016
- Content source:
Error processing SSI file


 ShareCompartir
ShareCompartir