
Trichuriasis
[Trichuris trichiura]
Causal Agent
The nematode (roundworm) Trichuris trichiura, also called the human whipworm.
Life Cycle
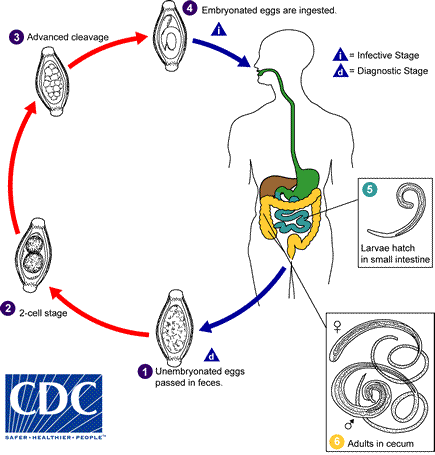
The unembryonated eggs are passed with the stool  . In the soil, the eggs develop into a 2-cell stage
. In the soil, the eggs develop into a 2-cell stage  , an advanced cleavage stage
, an advanced cleavage stage  , and then they embryonate
, and then they embryonate  ; eggs become infective in 15 to 30 days. After ingestion (soil-contaminated hands or food), the eggs hatch in the small intestine, and release larvae
; eggs become infective in 15 to 30 days. After ingestion (soil-contaminated hands or food), the eggs hatch in the small intestine, and release larvae  that mature and establish themselves as adults in the colon
that mature and establish themselves as adults in the colon  . The adult worms (approximately 4 cm in length) live in the cecum and ascending colon. The adult worms are fixed in that location, with the anterior portions threaded into the mucosa. The females begin to oviposit 60 to 70 days after infection. Female worms in the cecum shed between 3,000 and 20,000 eggs per day. The life span of the adults is about 1 year.
. The adult worms (approximately 4 cm in length) live in the cecum and ascending colon. The adult worms are fixed in that location, with the anterior portions threaded into the mucosa. The females begin to oviposit 60 to 70 days after infection. Female worms in the cecum shed between 3,000 and 20,000 eggs per day. The life span of the adults is about 1 year.
Geographic Distribution
The third most common round worm of humans. Worldwide, with infections more frequent in areas with tropical weather and poor sanitation practices, and among children. It is estimated that 800 million people are infected worldwide. Trichuriasis occurs in the southern United States.
Clinical Presentation
Most frequently asymptomatic. Heavy infections, especially in small children, can cause gastrointestinal problems (abdominal pain, diarrhea, rectal prolapse) and possibly growth retardation.
T. trichiura eggs.
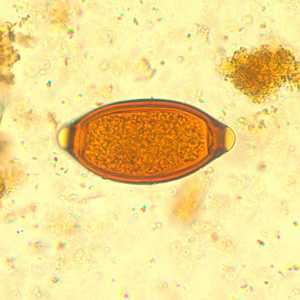
Figure A: Egg of T. trichiura in an iodine-stained wet mount.
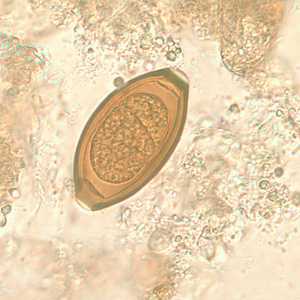
Figure B: Egg of T. trichiura in an unstained wet mount.
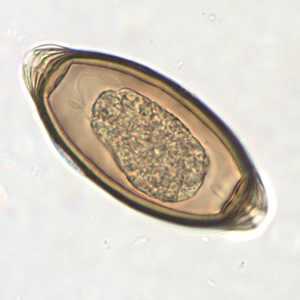
Figure C: Egg of T. trichiura in an unstained wet mount.
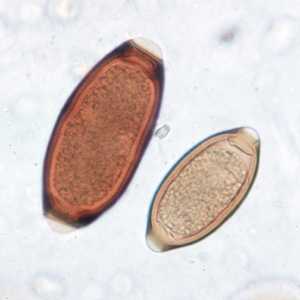
Figure D: Eggs of T. trichiura in a wet mount, showing variability in size in the species.
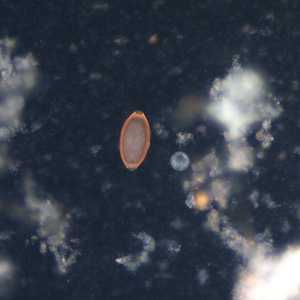
Figure E: Egg of T. trichiura viewed with UV microscopy.
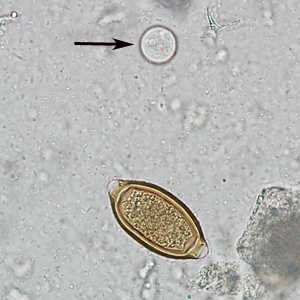
Figure F: Egg of T. trichiura in an unstained wet mount of stool. Notice also the presence of a cyst of Entamoeba coli (arrow).
Atypical T. trichiura eggs.
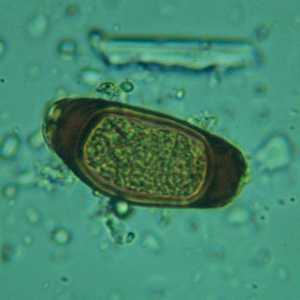
Figure A: Atypical egg of T. trichiura.
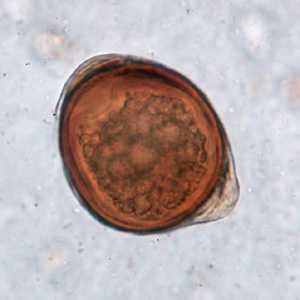
Figure B: Atypical egg of T. trichiura.
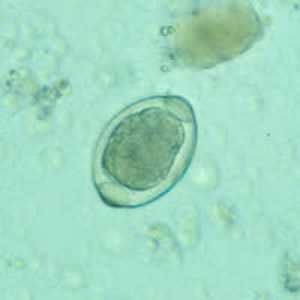
Figure C: Atypical egg of T. trichiura.
Cross-sections of T. trichiura stained with hematoxylin and eosin (H&E).
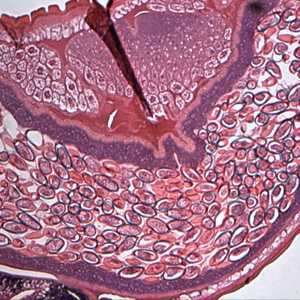
Figure A: Cross-section of a gravid female T. trichiura stained with Hamp;E, showing numerous eggs. Magnification at 100x. Image courtesy of the Oregon State Public Heath Laboratory.
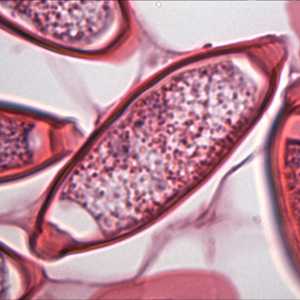
Figure B: Same specimen as in Figure A but at 1000x magnification, showing a close-up of one of the eggs.
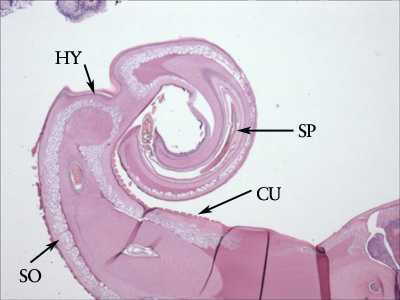
Figure C: Cross-section of the posterior end of an adult T. trichiura, from a colonoscopy specimen stained with Hamp;E. Note the presence of the thick cuticle with annulations (CU). Below the cuticle is the thin hypodermis (HY), and below the hypodermis is a layer of somatic muscle cells (SO). The presence of a spicule (SP) indicates the specimen is a male. Image courtesy of the Michael E. DeBakey V. A. Medical Center, Houston, TX.
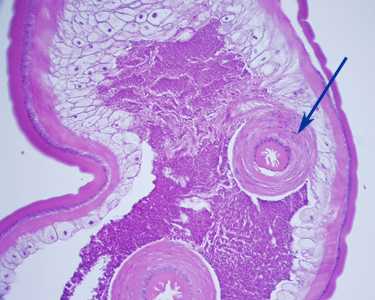
Figure D: Section of an adult T. trichiura, stained with H&E. Notice the thick-muscled cloaca (arrow). Image courtesy of Cambridge Health Alliance, Cambridge, MA.
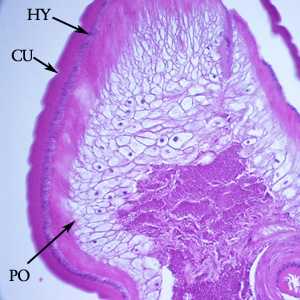
Figure E: Another image from the specimen in Figure D. Notice the thick cuticle with annulations (CU), a thin nucleate hypodermis (HY) and layers of polymyarian muscle cells (PO).
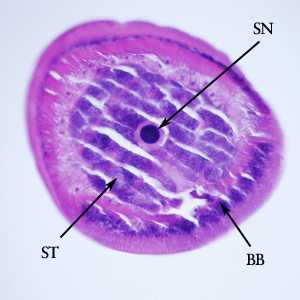
Figure F: Cross-section of the anterior end of the specimen in Figures D and E. Notice the bacillary band (BB), a stichocyte (ST) and stichosome nucleus (SN).
T. trichiura adults.
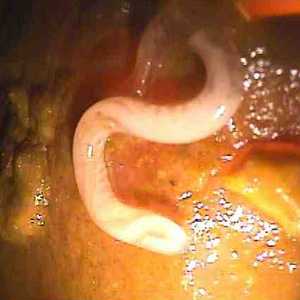
Figure A: Posterior end of an adult T. trichiura, taken during a colonoscopy. Image courtesy of Duke University Medical Center.
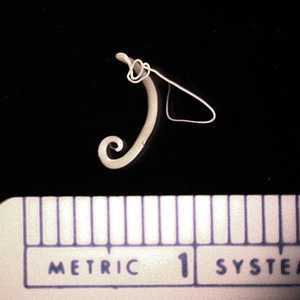
Figure B: Adult if T. trichiura removed during a colonoscopy.
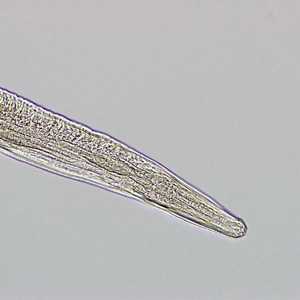
Figure C: Higher magnification of the anterior end of the specimen in Figure B.

Figure D: Higher magnification of the posterior end of the specimen in Figure B. Notice the prominent spicule.
Laboratory Diagnosis
Microscopic identification of whipworm eggs in feces is evidence of infection. Because eggs may be difficult to find in light infections, a concentration procedure is recommended. Because the severity of symptoms depend on the worm burden, quantification of the latter (e.g. with the Kato-Katz technique) can prove useful.
Examination of the rectal mucosa by proctoscopy (or directly in case of prolapses) can occasionally demonstrate adult worms.
Treatment Information
Whipworm is effectively treated with albendazole, mebendazole or ivermectin. Each drug needs to be taken for 3 days. Dosage guidelines are the same for children as for adults. Albendazole should be taken with food. Ivermectin should be taken with water on an empty stomach and the safety of ivermectin for children weighing less than 15 kg has not been established. Neither albendazole nor ivermectin is FDA-approved for treating whipworm.
| Drug | Dosage for adults and children |
|---|---|
| Albendazole | 400 mg orally for 3 days |
| Mebendazole | 100 mg orally twice a day for 3 days |
| Ivermectin | 200 mcg/kg/day orally for 3 days |
Albendazole
Oral albendazole is available for human use in the United States.
Note on Treatment in Pregnancy
Mebendazole
Mebendazole is available in the United States only through compounding pharmacies.
Note on Treatment in Pregnancy
Ivermectin
Oral ivermectin is available for human use in the United States.
Note on Treatment in Pregnancy
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir