
Toxoplasmosis
[Toxoplasma gondii]
Causal Agent
Toxoplasma gondii is a protozoan parasite that infects most species of warm blooded animals, including humans, and can cause the disease toxoplasmosis.
Life Cycle
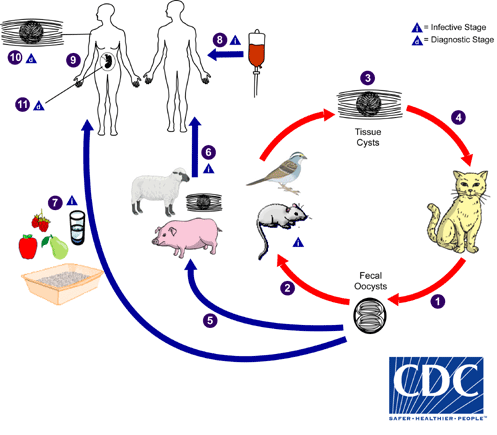
The only known definitive hosts for Toxoplasma gondii are members of family Felidae (domestic cats and their relatives). Unsporulated oocysts are shed in the cat’s feces  . Although oocysts are usually only shed for 1-2 weeks, large numbers may be shed. Oocysts take 1-5 days to sporulate in the environment and become infective. Intermediate hosts in nature (including birds and rodents) become infected after ingesting soil, water or plant material contaminated with oocysts
. Although oocysts are usually only shed for 1-2 weeks, large numbers may be shed. Oocysts take 1-5 days to sporulate in the environment and become infective. Intermediate hosts in nature (including birds and rodents) become infected after ingesting soil, water or plant material contaminated with oocysts  . Oocysts transform into tachyzoites shortly after ingestion. These tachyzoites localize in neural and muscle tissue and develop into tissue cyst bradyzoites
. Oocysts transform into tachyzoites shortly after ingestion. These tachyzoites localize in neural and muscle tissue and develop into tissue cyst bradyzoites  . Cats become infected after consuming intermediate hosts harboring tissue cysts
. Cats become infected after consuming intermediate hosts harboring tissue cysts  . Cats may also become infected directly by ingestion of sporulated oocysts. Animals bred for human consumption and wild game may also become infected with tissue cysts after ingestion of sporulated oocysts in the environment
. Cats may also become infected directly by ingestion of sporulated oocysts. Animals bred for human consumption and wild game may also become infected with tissue cysts after ingestion of sporulated oocysts in the environment  . Humans can become infected by any of several routes:
. Humans can become infected by any of several routes:
- eating undercooked meat of animals harboring tissue cysts
 .
. - consuming food or water contaminated with cat feces or by contaminated environmental samples (such as fecal-contaminated soil or changing the litter box of a pet cat)
 .
. - blood transfusion or organ transplantation
 .
. - transplacentally from mother to fetus
 .
.
In the human host, the parasites form tissue cysts, most commonly in skeletal muscle, myocardium, brain, and eyes; these cysts may remain throughout the life of the host. Diagnosis is usually achieved by serology, although tissue cysts may be observed in stained biopsy specimens  . Diagnosis of congenital infections can be achieved by detecting T. gondii DNA in amniotic fluid using molecular methods such as PCR
. Diagnosis of congenital infections can be achieved by detecting T. gondii DNA in amniotic fluid using molecular methods such as PCR  .
.
Geographic Distribution
Serologic prevalence data indicate that toxoplasmosis is one of the most common human infections throughout the world. A high prevalence of infection in France has been related to a preference for eating raw or undercooked meat, while a high prevalence in Central America has been related to the frequency of stray cats in a climate favoring survival of oocysts and soil exposure. The overall seroprevalence in the United States among adolescents and adults, as determined with specimens collected by the third National Health and Nutrition Examination Survey (NHANES III) between 1988 and 1994, was found to be 22.5%, with a seroprevalence among women of childbearing age (15 to 44 years) of 15%. In a more recent evaluation using data from NHANES 2009-2010, the overall age-adjusted T. gondii antibody seroprevalence among persons > 6 years of age was 12.4%, and among women 15–44 years of age was 9.1%.
Clinical Presentation
Acquired infection with Toxoplasma in immunocompetent persons is generally an asymptomatic infection. However, 10% to 20% of patients with acute infection may develop cervical lymphadenopathy and/or a flu-like illness. The clinical course is usually benign and self-limited; symptoms usually resolve within a few weeks to months. In rare cases ocular infection with visual loss can occur. Immunodeficient patients often have central nervous system (CNS) disease but may have retinochoroiditis, pneumonitis, or other systemic disease. In patients with AIDS, toxoplasmic encephalitis is the most common cause of intracerebral mass lesions and is thought to usually be caused by reactivation of chronic infection. Toxoplasmosis in patients being treated with immunosuppressive drugs may be due to either newly acquired or reactivated latent infection.
Congenital toxoplasmosis results from an acute primary infection acquired by the mother during pregnancy. The incidence and severity of congenital toxoplasmosis vary with the trimester during which infection was acquired. Because treatment of the mother may reduce the incidence of congenital infection and reduce sequelae in the infant, prompt and accurate diagnosis is important. Many infants with subclinical infection at birth will subsequently develop signs or symptoms of congenital toxoplasmosis. Ocular Toxoplasma infection, an important cause of retinochoroiditis in the United States, can be the result of congenital infection, or infection after birth. In congenital infection, patients are often asymptomatic until the second or third decade of life, when lesions develop in the eye.
Toxoplasma gondii tachyzoites.
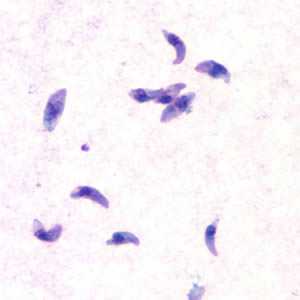
Figure A: Toxoplasma gondii tachyzoites, stained with Giemsa, from a smear of peritoneal fluid obtained from a laboratory-inoculated mouse.
Toxoplasma gondii cyst in brain tissue.
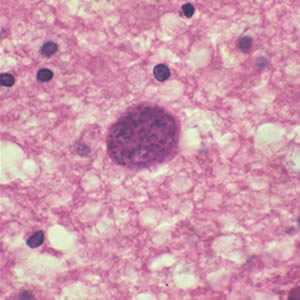
Figure A: Toxoplasma gondii cyst in brain tissue stained with hematoxylin and eosin.
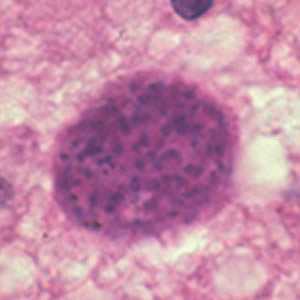
Figure B: Toxoplasma gondii cyst stained with hematoxylin and eosin.
Toxoplasma gondii cyst, unstained.
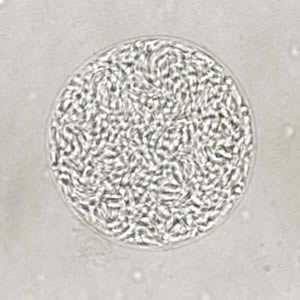
Figure A: Unstained cyst of T. gondii.
Toxoplasma gondii sporulated oocyst.
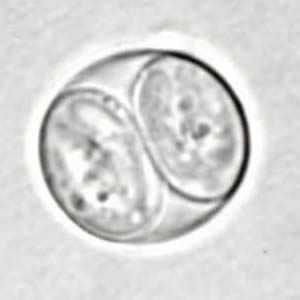
Figure A: Toxoplasma gondii sporulated oocyst in an unstained wet mount.
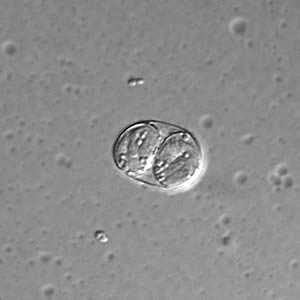
Figure B: Toxoplasma gondii sporulated oocyst in an unstained wet mount, viewed under differential interference contrast (DIC) microscopy.
Toxoplasma gondii unsporulated oocysts.
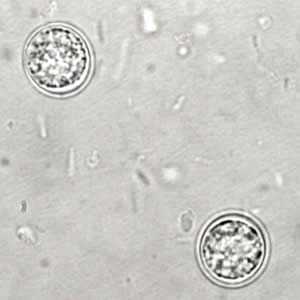
Figure A: Unsporulated T. gondii oocyst in an unstained wet mount.
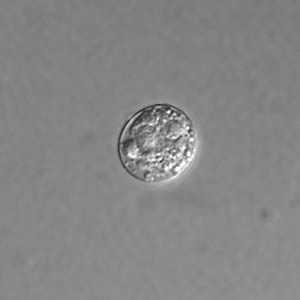
Figure B: Unsporulated oocyst of T. gondii in an unstained wet mount, viewed with differential interference contrast (DIC) microscopy.
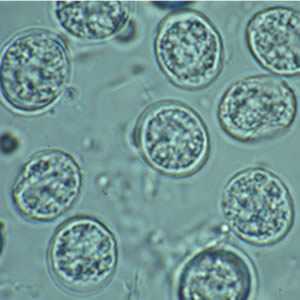
Figure C: T. gondii oocysts in a fecal floatation.
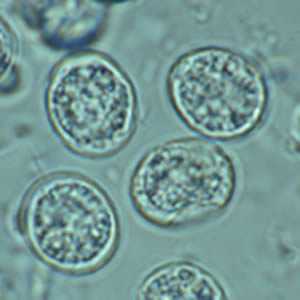
Figure D: Higher magnification of Figure C.
Toxoplasma gondii oocysts, UV microscopy.

Figure A: Toxoplasma gondii sporulated and unsporulated oocysts, UV fluorescence microscopy.
Ocular toxoplasmosis: Chorioretinitis.
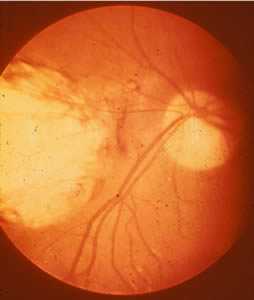
Figure A: Severe, active retinochoroiditis.
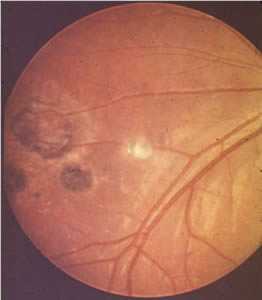
Figure B: Peripheral retinochoroiditis.
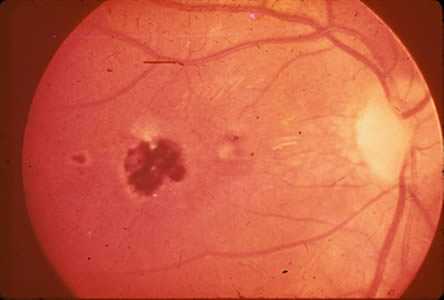
Figure C: Central, healed retinochoroiditis.
Laboratory Diagnosis
The diagnosis of toxoplasmosis may be documented by:
- Observation of parasites in patient specimens, such as bronchoalveolar lavage material from immunocompromised patients, or lymph node biopsy.
- Isolation of parasites from blood or other body fluids, by intraperitoneal inoculation into mice or tissue culture. The mice should be tested for the presence of Toxoplasma organisms in the peritoneal fluid 6 to 10 days post inoculation; if no organisms are found, serology can be performed on the animals 4 to 6 weeks post inoculation.
- Detection of parasite genetic material by PCR, especially in detecting congenital infections in utero.
- Serologic testing is the routine method of diagnosis.
Antibody Detection
The detection of Toxoplasma-specific antibodies is the primary diagnostic method to determine infection with Toxoplasma. Toxoplasma antibody detection tests are performed by a large number of laboratories with commercially available kits.
An algorithm for the immunodiagnosis of toxoplasmosis for individuals greater than one year of age is shown table below. The IFA and EIA tests for IgG and IgM antibodies are the tests most commonly used today. Persons should be initially tested for the presence of Toxoplasma-specific IgG antibodies to determine their immune status. A positive IgG titer indicates infection with the organism at some time. If more precise knowledge of the time of infection is necessary, then an IgG positive person should have an IgM test performed by a procedure with minimal nonspecific reactions, such as IgM-capture EIA. A negative IgM test essentially excludes recent infection, but a positive IgM test is difficult to interpret because Toxoplasma-specific IgM antibodies may be detected by EIA for as long as 18 months after acute acquired infection.
A major problem with Toxoplasma-specific IgM testing is lack of specificity. Two situations occur frequently: i) persons with a positive IgM but negative IgG, and ii) individuals with positive IgG and IgM results. In the first situation, a positive IgM result with a negative IgG result in the same specimen should be viewed with great suspicion; the patient's blood should be redrawn two weeks after the first and tested together with the first specimen. If the first specimen was drawn very early after infection, the patient should have highly positive IgG and IgM antibodies in the second sample. If the IgG is negative and the IgM is positive in both specimens, the IgM result should be considered to be a false positive and the patient should be considered to be not infected. In the second situation, a second specimen should be drawn and both specimens submitted together to a reference lab which employs a different IgM testing system for confirmation.
If the patient is pregnant, and IgG/IgM positive, an IgG avidity test should be performed. A high avidity result in the first 12 to 16 weeks of pregnancy (time dependent upon the commercial test kit) essentially rules out an infection acquired during gestation. A low IgG avidity result should not be interpreted as indicating recent infection, because some individuals have persistent low IgG avidity for many months after infection. Suspected recent infection in a pregnant woman should be confirmed prior to intervention by having samples tested at a toxoplasmosis reference laboratory. If the patient has clinical illness compatible with toxoplasmosis but the IgG titer is low, a follow-up titer two to three weeks later should show an increase in antibody titer if the illness is due to acute toxoplasmosis, assuming the host is not severely immunocompromised.
| IgG Result | IgM Result | Report/interpretation for humans* |
|---|---|---|
| Negative | Negative | No serological evidence of infection with Toxoplasma. |
| Negative | Equivocal | Possible early acute infection or false-positive IgM reaction. Obtain a new specimen for IgG and IgM testing. If results for the second specimen remain the same, the patient is probably not infected with Toxoplasma. |
| Negative | Positive | Possible acute infection or false-positive IgM result. Obtain a new specimen for IgG and IgM testing. If results for the second specimen remain the same, the IgM reaction is probably a false-positive. |
| Equivocal | Negative | Indeterminate: obtain a new specimen for testing or retest this specimen for IgG in a different essay. |
| Equivocal | Equivocal | Indeterminate: obtain a new specimen for both IgG and IgM testing. |
| Equivocal | Positive | Possible acute infection with Toxoplasma. Obtain a new specimen for IgG and IgM testing. If results for the second specimen remain the same or if the IgG becomes positive, both specimens should be sent to a reference laboratory with experience in diagnosis of toxoplasmosis for further testing. |
| Positive | Negative | Infected with Toxoplasma for six months or more. |
| Positive | Equivocal | Infected with Toxoplasma for probably more than 1 year or false-positive IgM reaction. Obtain a new specimen for IgM testing. If results with the second specimen remain the same, both specimens should be sent to a reference laboratory with experience in the diagnosis of toxoplasmosis for further testing. |
| Positive | Positive | Possible recent infection within the last 12 months, or false-positive IgM reaction. Send the specimen to a reference laboratory with experience in the diagnosis of toxoplasmosis for further testing. |
- *except infants
Newborn infants suspected of congenital toxoplasmosis should be tested by both an IgM- and an IgA-capture EIA. Detection of Toxoplasma-specific IgA antibodies is more sensitive than IgM detection in congenitally infected babies. None of the current commercial assays offered in the United States have been cleared by the Food and Drug Administration for in vitro diagnostic use for infants; consequently, all specimens from neonates suspected of having congenital toxoplasmosis should be sent to the Toxoplasma Serology Laboratory, Palo Alto, CA which has the most experience with infant testing.
Serological determination of active central nervous system toxoplasmosis in immunocompromised patients is not possible at this time. Toxoplasma-specific IgG antibody levels in AIDS patients often are low to moderate, but occasionally no specific IgG antibodies can be detected. Tests for IgM antibodies are generally negative.
Several commercial kits for Toxoplasma serologic testing are available. However, the sensitivity and specificity of these kits may vary widely from one commercial brand to another. This is of concern because serology results can influence decisions on continuation or termination of pregnancies.
References:
- NCCLS. Clinical Use and Interpretation of Serologic Tests for Toxoplasma gondii; Approved Guideline. NCCLS document M36-A [ISBN 1-56238-523-2]. NCCLS, 940 West Valley Road, Suite 1400, Wayne, PA 19087-1898 USA, 2004.
- McAuley JM, Jones JL, Singh AM. Toxoplasma. In: Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual of Clinical Microbiology. 11th ed. Washington, D.C.: American Society for Microbiology; 2015. p. 2373--2386.
- Remington JS, McLeod R, Wilson CB, Desmonts G. Toxoplasmosis. In: Remington JS, Klein JO, editors. Infectious Diseases of the Fetus and Newborn Infant. 7th ed. Philadelphia, PA: The WB Saunders Co.; 2011. p. 918-1041.
CDC 1998 Toxoplasma Human Serum Panel
To help evaluate the accuracy of commercial Toxoplasma antibody test kits, CDC created a Toxoplasma serum panel, the 'CDC 1998 Toxoplasma Human Serum Panel,' that contains known positive and negative sera. FDA now requires that any new commercial Toxoplasma test kit performs adequately based on results obtained using this panel. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4902a5.htm
A panel of 100 human specimens (1.0 ml each) has been assembled to include samples which are Toxoplasma antibody negative, Toxoplasma-specific IgG positive but IgM negative, Toxoplasma-specific IgG and IgM positive and Toxoplasma-specific IgG and IgA positive. Specimens with varying levels of reactivity were included. The panel was tested by the Toxoplasma Serology Laboratory, Palo Alto Research Institute, Palo Alto, CA, for IgG, IgM, and IgA by the Sabin-Feldman Dye Test, the IgM-ELISA, the IgA-ELISA, and the differential agglutination (AC/HS) tests. The panel has also been tested at the Centers for Disease Control and Prevention (CDC) by IFA-IgG and the CDC EIA-IgM.
This panel is available for purchase from CDC by device manufacturers / U.S. distributors for Toxoplasma human antibody detection assays. After the panel is tested with a device, the results will be sent to CDC for analysis. The submitter will receive a written analysis from CDC including sensitivity and specificity rates for each immunoglobulin subclass tested, limited results of IgG reproducibility, graphs of device positive results vs. standard tests for each immunoglobulin subclass tested, and information regarding IgM and IgA results vs. time post-infection for both the device and the standard tests.
Cost: $5000.
If you wish to purchase the panel, please fax or e-mail a letter on company letterhead stating that you wish to obtain the 'CDC Toxoplasma 1998 Human Serum Panel' to:
DPDx Team
Division of Parasitic Diseases and Malaria
Centers for Disease Control and Prevention
Fax: 404-718-4195
Email: DPDx@cdc.gov
Treatment Information
Treatment of immunocompetent adults with lymphadenopathic toxoplasmosis is rarely indicated; this form of the disease is usually self-limited. If visceral disease is clinically evident or symptoms are severe or persistent, treatment may be indicated for 2 to 4 weeks. Treatment for ocular diseases should be based on a complete ophthalmologic evaluation. The decision to treat ocular disease is dependent on numerous parameters including acuteness of the lesion, degree of inflammation, visual acuity, and lesion size, location, and persistance (for example, "classic therapy" for ocular toxoplasmosis, adults: pyrimethamine 100 mg for 1 day as a loading dose, then 25 to 50 mg per day, plus sulfadiazine 1 gram four times per day, plus folinic acid (leucovorin) 5-25 mg with each dose of pyrimethamine; pediatric dose: pyrimethamine 2 mg/kg first day then 1 mg/kg each day, plus sulfadiazine 50 mg/kg two times per day, plus folinic acid (leucovorin) 7.5 mg per day) for 4 to 6 weeks followed by reevaluation of the patient's condition. Leucovorin protects the bone marrow from the toxic effects of pyrimethamine. If the patient has a hypersensitivity reaction to sulfa drugs, pyrimethamine plus clindamycin can be used instead. The fixed combination of trimethoprim with sulfamethoxazole has been used as an alternative, as well as other drugs such as atovaquone and pyrimethamine plus azithromycin, which have not been extensively studied (see: Montoya JG, Boothroyd JC, Kovacs JA. Toxoplasma gondii in Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 7th, Edition, 2010 (8th edition planned for 2015). Mandell GL, Bennett JE, Dolin R, Eds. Churchill Livingstone Elsevier, Philadelphia, PA.; and de-la-Torre A, Stanford M, Curi A, Jaffe GJ, Gomez-Marin JE. Therapy for ocular toxoplasmosis. Ocul Immunol Inflamm. 2011;19:314-20). Corticosteroids are sometimes prescribed in addition to antiparasitic agents.
Management of maternal and fetal infection varies depending on the treatment center. In general, spiramycin is recommended (for the first and early second trimesters) or pyrimethamine/sulfadiazine and leucovorin (for late second and third trimesters) for women with acute T. gondii infection diagnosed at a reference laboratory during gestation. PCR is often performed on the amniotic fluid at 18 gestation weeks to determine if the infant is infected. If the infant is likely to be infected, then treatment with drugs such as pyrimethamine, sulfadiazine, and leucovorin is typical. Congenitally infected newborns are generally treated with pyrimethamine, a sulfonamide, and leucovorin for 1 year (see Montoya JG, Liesenfeld O. Toxoplasmosis![]() . Lancet. 2004 Jun 12;363:1965-1976; for additional information regarding management in pregnant women, see Montoya JG, Remington JS. Management of Toxoplasma gondii infection in pregnancy. Clin Infect Dis 2008;47:554-566).
. Lancet. 2004 Jun 12;363:1965-1976; for additional information regarding management in pregnant women, see Montoya JG, Remington JS. Management of Toxoplasma gondii infection in pregnancy. Clin Infect Dis 2008;47:554-566).
Persons with AIDS who develop active toxoplasmosis (usually toxoplasmic enchephalitis) need treatment that must be taken until a significant immunologic improvement is achieved as a result of antiretroviral therapy. See the toxoplasmosis sections in the Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America for adults/adolescents: https://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-oi-prevention-and-treatment-guidelines/0![]() , and from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics for children https://aidsinfo.nih.gov/guidelines/html/5/pediatric-oi-prevention-and-treatment-guidelines/0
, and from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics for children https://aidsinfo.nih.gov/guidelines/html/5/pediatric-oi-prevention-and-treatment-guidelines/0![]() .
.
More on: Guidelines for the Prevention and Treatment of Opportunistic Infection in HIV-Exposed and HIV-Infected Children![]() .
.
Pyrimethamine
Pyrimethamine is available for human use in the United States.
Note on Treatment in Pregnancy
Sulfadiazine
Sulfadiazine is available for human use in the United States.
Note on Treatment in Pregnancy
Clindamycin
Clindamycin is available for human use in the United States.
Note on Treatment in Pregnancy
Note on Treatment During Lactation
Note on Treatment in Pediatric Patients
Trimethoprim–sulfamethoxazole
Trimethoprim–sulfamethoxazole (TMP–SMX) is available for human use in the United States.
Note on Treatment in Pregnancy
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir