
Opisthorchiasis
[Opisthorchis felineus] [Opisthorchis viverrini]
Causal Agents
Trematodes (flukes) Opisthorchis viverrini (Southeast Asian liver fluke) and O. felineus (cat liver fluke).
Life Cycle
The adult flukes deposit fully developed eggs that are passed in the feces . After ingestion by a suitable snail (first intermediate host)
. After ingestion by a suitable snail (first intermediate host) , the eggs release miracidia
, the eggs release miracidia , which undergo in the snail several developmental stages (sporocysts
, which undergo in the snail several developmental stages (sporocysts , rediae
, rediae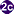 , cercariae
, cercariae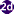 ). Cercariae are released from the snail
). Cercariae are released from the snail and penetrate freshwater fish (second intermediate host), encysting as metacercariae in the muscles or under the scales
and penetrate freshwater fish (second intermediate host), encysting as metacercariae in the muscles or under the scales . The mammalian definitive host (cats, dogs, and various fish-eating mammals including humans) become infected by ingesting undercooked fish containing metacercariae. After ingestion, the metacercariae excyst in the duodenum
. The mammalian definitive host (cats, dogs, and various fish-eating mammals including humans) become infected by ingesting undercooked fish containing metacercariae. After ingestion, the metacercariae excyst in the duodenum and ascend through the ampulla of Vater into the biliary ducts, where they attach and develop into adults, which lay eggs after 3 to 4 weeks
and ascend through the ampulla of Vater into the biliary ducts, where they attach and develop into adults, which lay eggs after 3 to 4 weeks . The adult flukes (O. viverrini: 5 mm to 10 mm by 1 mm to 2 mm; O. felineus: 7 mm to 12 mm by 2 mm to 3 mm) reside in the biliary and pancreatic ducts of the mammalian host, where they attach to the mucosa.
. The adult flukes (O. viverrini: 5 mm to 10 mm by 1 mm to 2 mm; O. felineus: 7 mm to 12 mm by 2 mm to 3 mm) reside in the biliary and pancreatic ducts of the mammalian host, where they attach to the mucosa.
Geographic Distribution
Opisthorchis viverrini is found mainly in northeast Thailand, Laos, and Kampuchea. Opisthorchis felineus is found mainly in Europe and Asia, including the former Soviet Union.
Clinical Presentation
Most infections are asymptomatic. In mild cases, manifestations include dyspepsia, abdominal pain, diarrhea or constipation. With infections of longer duration, the symptoms can be more severe, and hepatomegaly and malnutrition may be present. In rare cases, cholangitis, cholecystitis, and chlolangiocarcinoma may develop. In addition, infections due to Opisthorchis felineus may present an acute phase resembling Katayama fever (schistosomiasis), with fever, facial edema, lymphadenopathy, arthralgias, rash, and eosinophilia. Chronic forms of Opisthorchis felineus infections present the same manifestations as Opisthorchis viverrini, with in addition involvement of the pancreatic ducts.
Eggs of Opisthorchis spp. in wet mounts.
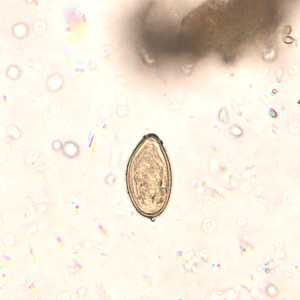
Figure A: Egg of O. viverrini in an unstained wet mount of concentrated stool. Image taken at 400x magnification.
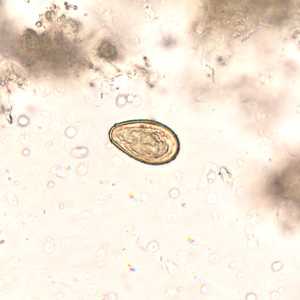
Figure B: Egg of O. viverrini in an unstained wet mount of concentrated stool. Image taken at 400x magnification
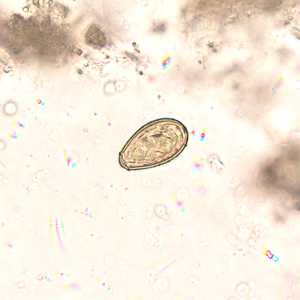
Figure C: Egg of O. viverrini in an unstained wet mount of concentrated stool. Image taken at 400x magnification.
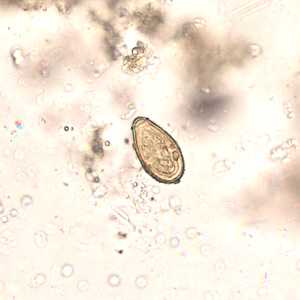
Figure D: Egg of O. viverrini in an unstained wet mount of concentrated stool. Image taken at 400x magnification.
Adults of Opisthorchis spp.
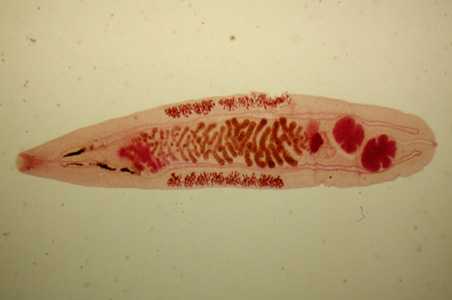
Figure A: Adult of O. felineus. Image courtesy of the Web Atlas of Medical Parasitology and the Korean Society for Parasitology.
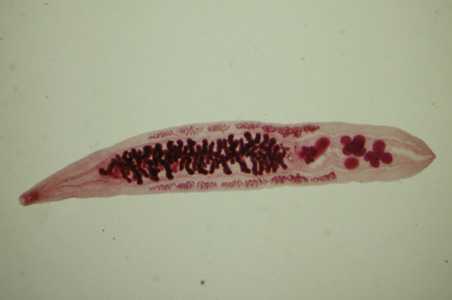
Figure B: Adult of O. viverrini. Image courtesy of the Web Atlas of Medical Parasitology and the Korean Society for Parasitology.
Intermediate hosts of Opisthorchis spp.

Figure A: Bithynia sp., a common intermediate host of Opisthorchis spp. Image courtesy of Michal Maňas.
Laboratory Diagnosis
Diagnosis is based on microscopic identification of eggs in stool specimens. However, the eggs of Opisthorchis are practically indistinguishable from those of Clonorchis.
Treatment Information
Praziquantel, adults, 75mg/kg/day orally, three doses per day for 2 days; the pediatric dosage is the same. Praziquantel should be taken with liquids during meals.
Alternative:
Albendazole* is an alternative drug; the dosage is 10mg/kg/day for 7 days. The pediatric dosage is the same. Albendazole should be taken with food; a fatty meal increases the bioavailablility.
* Not FDA-approved for this indication
Praziquantel
Oral praziquantel is available for human use in the United States.
Note on Treatment in Pregnancy
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: April 27, 2017
- Page last updated: May 3, 2017
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir