
Microsporidiosis
[Anncaliia spp.] [Encephalitozoon cuniculi] [Encephalitozoon hellem] [Encephalitozoon intestinalis (syn. Septata intestinalis)] [Tubulinosema acridophagus] [Enterocytozoon bieneusi] [Nosema spp.] [Pleistophora sp.] [Trachipleistophora spp.] [Vittaforma corneae (syn. Nosema corneum)]
Causal Agents
The microsporidia are a group of obligate intracellular parasitic fungi. Historically, they have been treated among the protozoa, and as such are often still managed by diagnostic parasitology laboratories. To date, more than 1,200 species belonging to 143 genera have been described as parasites infecting a wide range of vertebrate and invertebrate hosts. Microsporidia, are characterized by the production of resistant spores that vary in size, depending on the species. They possess a unique organelle, the polar tubule or polar filament, which is coiled inside the spore as demonstrated by its ultrastructure. The microsporidia spores of species associated with human infection measure from 1 to 4 µm and that is a useful diagnostic feature. There are at least 15 microsporidian species that have been identified as human pathogens: Anncaliia (formerly Brachiola) algerae, A. connori, A. vesicularum, Encephalitozoon cuniculi, E. hellem, E. intestinalis, Enterocytozoon bieneusi Microsporidium ceylonensis, M. africanum, Nosema ocularum, Pleistophora sp., Trachipleistophora hominis, T. anthropophthera, Vittaforma corneae, and Tubulinosema acridophagus. Encephalitozoon intestinalis was previously named Septata intestinalis, but it was reclassified as Encephalitozoon intestinalis based on its similarity at the morphologic, antigenic, and molecular levels to other species of this genus. Based on recent data it is now known that some domestic and wild animals may be naturally infected with the following microsporidian species: E. cuniculi, E. intestinalis, E. bieneusi. Birds, especially parrots (parakeets, love birds, budgies) are naturally infected with E. hellem. E. bieneusi and V. corneae have been identified in surface waters, and spores of Nosema sp. (likely A. algerae) have been identified in ditch water. Tubulinosema acridophagus, an insect parasite, has recently (2012) been implicated in two cases of disseminated microsporidiosis.
Life Cycle
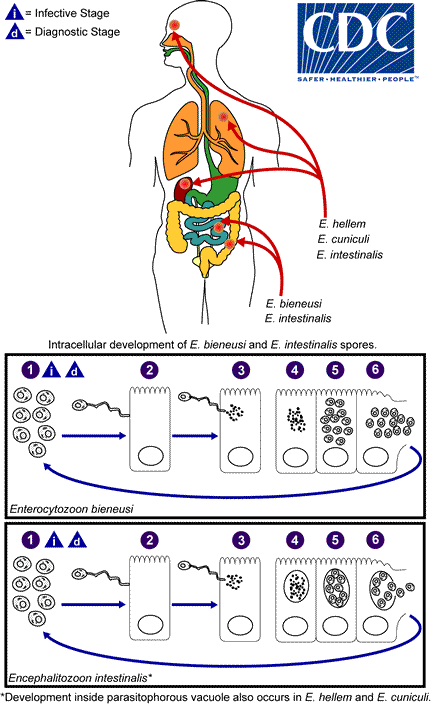
The infective form of microsporidia is the resistant spore and it can survive for a long time in the environment  . The spore extrudes its polar tubule and infects the host cell
. The spore extrudes its polar tubule and infects the host cell  . The spore injects the infective sporoplasm into the eukaryotic host cell through the polar tubule
. The spore injects the infective sporoplasm into the eukaryotic host cell through the polar tubule  . Inside the cell, the sporoplasm undergoes extensive multiplication either by merogony (binary fission) or schizogony (multiple fission)
. Inside the cell, the sporoplasm undergoes extensive multiplication either by merogony (binary fission) or schizogony (multiple fission)  . This development can occur either in direct contact with the host cell cytoplasm (e.g., E. bieneusi) or inside a vacuole termed parasitophorous vacuole (e.g., E. intestinalis). Either free in the cytoplasm or inside a parasitophorous vacuole, microsporidia develop by sporogony to mature spores
. This development can occur either in direct contact with the host cell cytoplasm (e.g., E. bieneusi) or inside a vacuole termed parasitophorous vacuole (e.g., E. intestinalis). Either free in the cytoplasm or inside a parasitophorous vacuole, microsporidia develop by sporogony to mature spores  . During sporogony, a thick wall is formed around the spore, which provides resistance to adverse environmental conditions. When the spores increase in number and completely fill the host cell cytoplasm, the cell membrane is disrupted and releases the spores to the surroundings
. During sporogony, a thick wall is formed around the spore, which provides resistance to adverse environmental conditions. When the spores increase in number and completely fill the host cell cytoplasm, the cell membrane is disrupted and releases the spores to the surroundings  . These free mature spores can infect new cells thus continuing the cycle.
. These free mature spores can infect new cells thus continuing the cycle.
Geographic Distribution
Microsporidia are being increasingly recognized as opportunistic infectious agents worldwide.
Clinical Presentation
Human microsporidiosis represents an important and rapidly emerging opportunistic disease, occurring mainly, but not exclusively, in severely immunocompromised patients with AIDS. Additionally, cases of microsporidiosis in immunocompromised persons not infected with HIV as well as in immunocompetent persons also have been reported. The clinical manifestations of microsporidiosis are very diverse, varying according to the causal species with diarrhea being the most common.
| Microsporidian species | Clinical manifestation |
|---|---|
| Anncaliia algerae | Keratoconjunctivitis, skin and deep muscle infection |
| Enterocytozoon bieneusi* | Diarrhea, acalculous cholecystitis |
| Encephalitozoon cuniculi and Encephalitozoon hellem | Keratoconjunctivitis, infection of respiratory and genitourinary tract, disseminated infection |
| Encephalitozoon intestinalis (syn. Septata intestinalis) | Infection of the GI tract causing diarrhea, and dissemination to ocular, genitourinary and respiratory tracts |
| Microsporidium (M. ceylonensis and M. africanum) | Infection of the cornea |
| Nosema sp. (N. ocularum), Anncaliia connori | Ocular infection |
| Pleistophora sp. | Muscular infection |
| Trachipleistophora anthropophthera | Disseminated infection |
| Trachipleistophora hominis | Muscular infection, stromal keratitis, (probably disseminated infection) |
| Tubulinosema acridophagus | Disseminated infection |
| Vittaforma corneae (syn. Nosema corneum) | Ocular infection, urinary tract infection |
*Two reports of E. bieneusi in respiratory samples have also been published, one in 1992 and the other in 1997.
Electron micrographs of microsporidia.
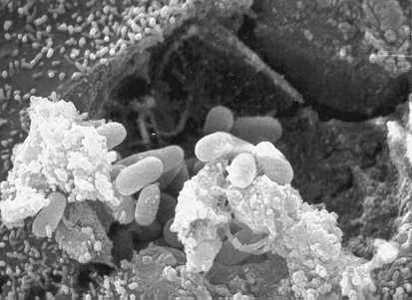
Figure A: Scanning electron micrograph showing an eukaryotic cell bursting and releasing spores of Encephalitozoon hellem to the extracellular medium.
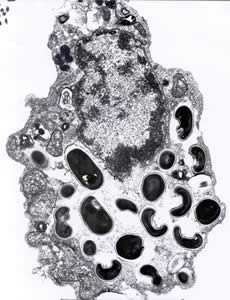
Figure B: Electron micrograph of an eukaryotic cell with Encephalitozoon intestinalis spores and developing forms inside a septated parasitophorous vacuole. The vacuole is a characteristic feature of this microsporidian species.
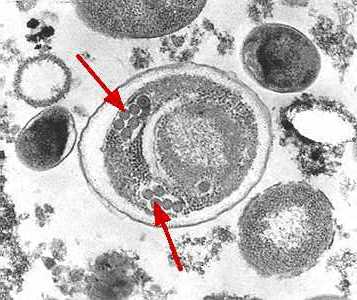
Figure C: Electron micrograph of an Enterocytozoon bieneusi spore. Arrows indicate the double rows of polar tubule coils in cross section which characterize a mature E. bieneusi spore.
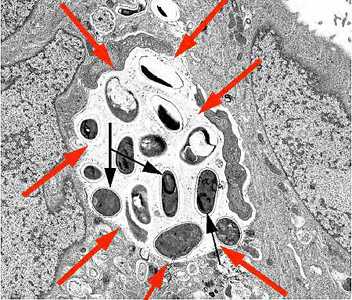
Figure D: Transmission electron micrograph of E. intestinalis depicting developing forms inside a parasitophorous vacuole (red arrows) with mature spores (black arrows).
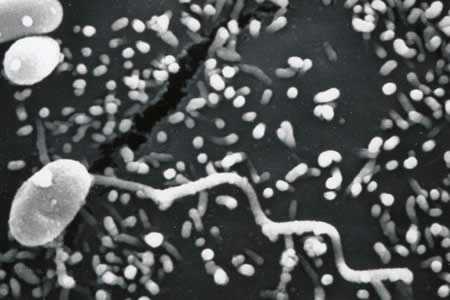
Figure E: Scanning electron micrograph of a microsporidian spore with an extruded polar tubule inserted into a eukaryotic cell. The spore injects the infective sporoplasms through its polar tubule.
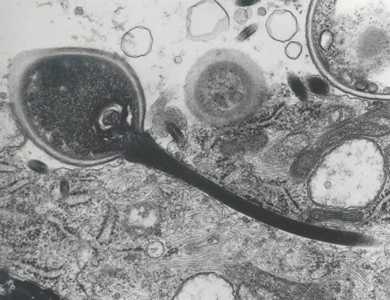
Figure F: Transmission electron micrograph of a microsporidian spore with an extruded polar tubule inserted into a eukaryotic cell. The spore injects the infective sporoplasms through its polar tubule. Figure courtesy of Dr. Massimo Scaglia, Laboratory of Clinical Parasitology, Institute of Infectious Diseases, University-IRCCS San Matteo, Pavia, Italy.
Microsporidia stained with Chromotrope 2R and Gram chromotrope stains.
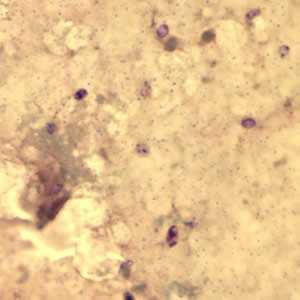
Figure A: Encephalitozoon cuniculi spores stained with Gram Chromotrope.
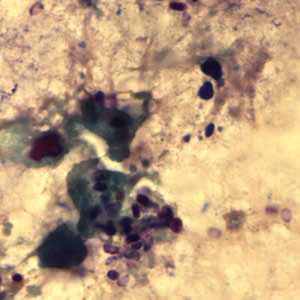
Figure B: Encephalitozoon cuniculi spores stained with Gram Chromotrope.
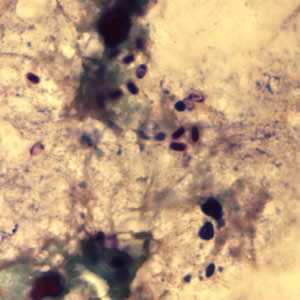
Figure C: Encephalitozoon cuniculi spores stained with Gram Chromotrope.
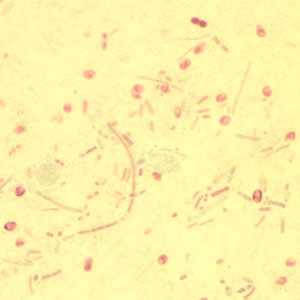
Figure D: Enterocytozoon bieneusi spores stained with Chromotrope 2R.
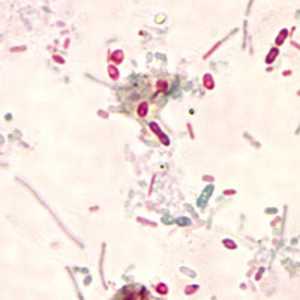
Figure E: Unidentified microsporidia stained with Chromotrope 2R.
Encephalitozoon cuniculi in urine and kidney biopsy specimens, stained with Ryan's modified trichrome stain.
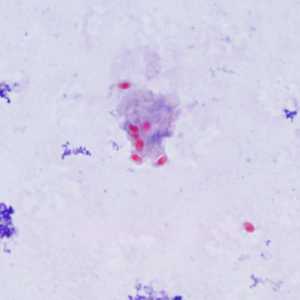
Figure A: Spores of E. cuniculi from urine stained with Ryan's modified trichrome (Trichrome blue).
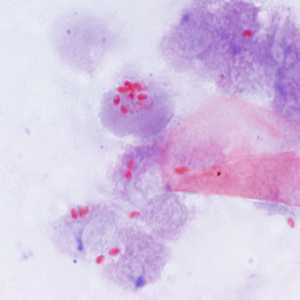
Figure B: Spores of E. cuniculi from urine stained with Ryan's modified trichrome (Trichrome blue).
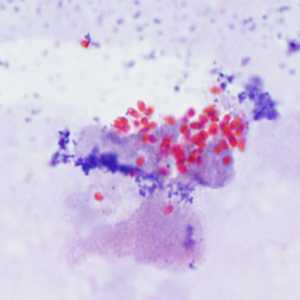
Figure C: Spores of E. cuniculi from urine stained with Ryan's modified trichrome.
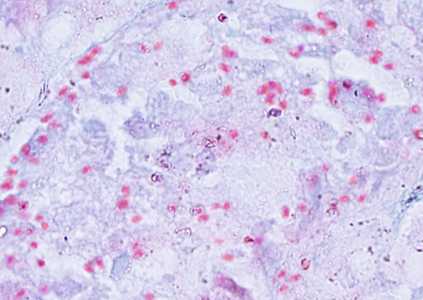
Figure D: Spores of E. cuniculi in a kidney biopsy specimen stained with Ryan's modified trichrome.
Tubulinosema acridophagus in bronchoalveolar lavage (BAL) specimens, stained with Chromotrope 2R stain.
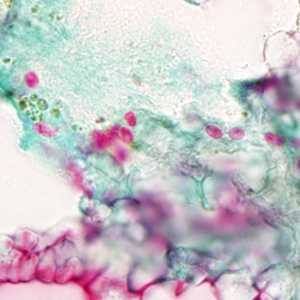
Figure A: Spores of T. acridophagus in BAL specimens, stained with Chromotrope 2R stain.
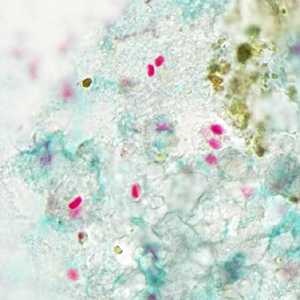
Figure B: Spores of T. acridophagus in BAL specimens, stained with Chromotrope 2R stain.
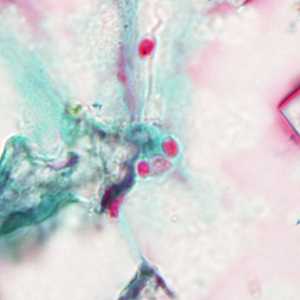
Figure C: Spores of Tubulinosema acridophagus in (BAL) specimens, stained with Chromotrope 2R stain.
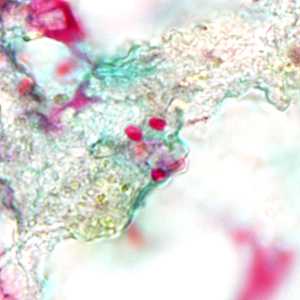
Figure D: Spores of Tubulinosema acridophagus in (BAL) specimens, stained with Chromotrope 2R stain.
Ocular microsporidiosis stained with trichrome and Giemsa.
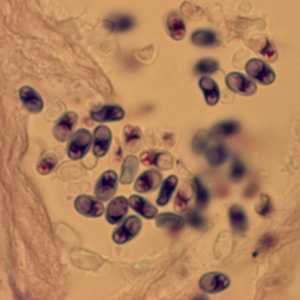
Figure A: Microsporidia spores from a corneal section, stained with Giemsa.
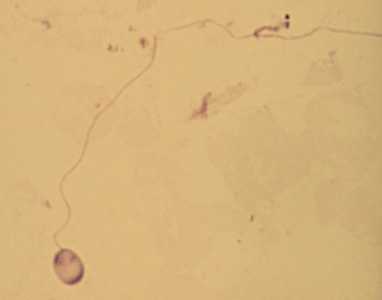
Figure B: A single microsporidia spore from the same case as Figure A, stained with Giemsa. In this image, the polar tubule has been extruded.
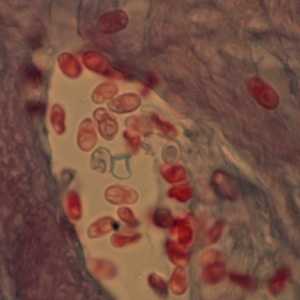
Figure C: Microsporidia spores from a corneal section, stained with trichrome.
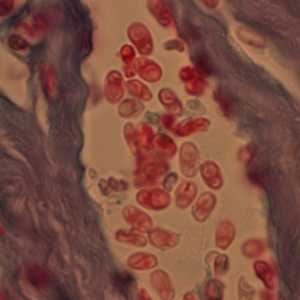
Figure D: Microsporidia spores from a corneal section, stained with trichrome.
Encephalitozoon intestinalis stained with Calcofluor white.
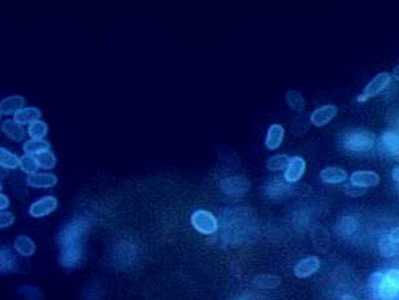
Figure A: Encephalitozoon intestinalis stained with Calcofluor white.
Monoclonal antibody-based immunofluorescence identification of Encephalitozoon hellem.
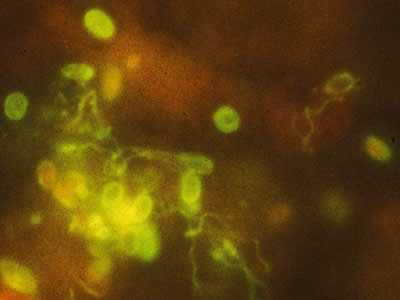
Figure A: Monoclonal antibody-based immunofluorescence identification of Encephalitozoon hellem.
Laboratory Diagnosis
There are several methods for diagnosing microsporidia:
Microscopy
Light microscopic examination of the stained clinical smears, especially the fecal samples, is an inexpensive method of diagnosing microsporidial infections even though it does not allow identification of microsporidia to the species level. The most widely used staining technique is the Chromotrope 2R method or its modifications. This technique stains the spore and the spore wall a bright pinkish red. Often, a belt-like stripe, which also stains pinkish red, is seen in the middle of the spore. This technique, however, is lengthy and time consuming and requires about 90 min. A recently developed “Quick-Hot Gram Chromotrope technique” however, cuts down the staining time to less than 10 min and provides a good differentiation from the lightly stained background fecal materials so that the spores stand out for easy visualization. The spores stain dark violet and the belt-like stripe is enhanced. In some cases dark staining Gram positive granules are also clearly seen. Chemofluorescent agents such as Calcofluor white are also useful in the quick identification of spores in fecal smears. The spores measure from 0.8 to 1.4 µm in the case of Enterocytozoon bieneusi, and 1.5 to 4 µm in Anncaliia algerae, Encephalitozoon spp., Vittaforma corneae, and Nosema spp.
Transmission electron microscopy (TEM) is still the gold standard and is necessary for the identification of the microsporidian species. However, TEM is expensive, time consuming, and not feasible for routine diagnosis.
Immunofluorescence Assays (IFA)
IFAs are available for microsporidia using monoclonal and/or polyclonal antibodies.
Molecular Methods (PCR)
The CDC offers molecular identification of Enterocytozoon bieneusi, Encephalitozoon intestinalis, Encephalitozoon hellem and Encephalitozoon cuniculi using species-specific polymerase chain reaction (PCR) assays. Molecular identification of other microsporidia species can be attempted using genera-specific primers and sequencing analysis on a case-by-case basis.
References:
- Visvesvara GS, Da Silva AJ, Croppo JP, Pieniazek NJ, Leitch GJ, Ferguson D, De Moura H, Wallace S, Slemenda SB, Tyrrell I, Moore DF, Meador J. In Vitro Culture and Serologic and Molecular Identification of Septata intestinalis Isolated from Urine of a Patient with AIDS. Journal of Clinical Microbiology, Apr. 1995, p. 930–936 Vol. 33, No. 4
- Da Silva AJ, Schwartz DA, Visvesvara GS, De Moura H, Slemenda SB, Pieniazek NJ. Sensitive PCR Diagnosis of Infections by Enterocytozoon bieneusi (Microsporidia) Using Primers Based on the Region Coding for Small-Subunit rRNA. Journal of Clinical Microbiology, Apr. 1996, p. 986–987 Vol. 34, No. 4
- Del Aguila C, Croppo GP, Moura H, Da Silva AJ, Leitch GJ, Moss DM, Wallace S, Slemenda SB, Pieniazek NJ, Visvesvara GS. Ultrastructure, Immunofluorescence, Western Blot, and PCR Analysis of Eight Isolates of Encephalitozoon (Septata) intestinalis Established in Culture from Sputum and Urine Samples and Duodenal Aspirates of Five Patients with AIDS. Journal of Clinical Microbiology May 1998, p. 1201–1208 Vol. 36, No. 5
- Meissner EG, Bennett JE, Qvarnstrom Y, da Silva A, Chu EY, Tsokos M and Gea-Banacloche J. Disseminated Microsporidiosis in an Immunosuppressed Patient. Emerging Infectious Diseases July 2012, Vol. 18, No. 7, p. 1155-1158.
Treatment Information
For gastrointestinal infections caused by Enterocytozoon bieneusi, fumagillin 20 mg orally three times daily is the only drug with proven efficacy. However, its use is associated with severe thrombocytopenia in 30-50% of patients, which is reversible upon discontinuation of treatment, and the drug is not currently available in the United States.
For disseminated (not ocular) and intestinal infection attributed to microsporidia other than E. bieneusi and Vittaforma corneae, the drug of choice is albendazole 400 mg orally twice daily. Treatment should continue until immune reconstitution has been maintained for at least 6 months. Itraconazole 400 mg orally daily plus albendazole 400 mg orally twice daily may have activity for disseminated disease attributed to Trachipleistophora or Anncaliia.
For ocular infection, the treatment of choice is topical fumagillin bicylohexylammonium (Fumidil B) 3 mg/mL in saline (fumagillin 70 µg/mL) eye drops: two drops every 2 hours for 4 days, then two drops four times daily (investigational use only in United States) plus albendazole 400 mg orally twice daily for management of systemic infection.
Initiation or optimization of antiretroviral therapy is the cornerstone of treatment of microsporidiosis in HIV-infected patients. Immune restoration to CD4 cell count >100 cells/mm3 is associated with resolution of symptoms of enteric microsporidiosis. Management of severe dehydration, malnutrition, and wasting with fluid support and nutritional supplementation should be provided. Use of antimotility agents for diarrhea control can be considered in infected adults.
Although albendazole is likely less effective against E. bieneusi, there are reports of success with albendazole therapy in immunosuppressed patients.
For more information, please visit http://aidsinfo.nih.gov/guidelines![]() .
.
* This drug is approved by the FDA, but considered investigational for this purpose.
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir