Mansonellosis
[Mansonella ozzardi] [Mansonella perstans] [Mansonella streptocerca]
Causal Agents
Filarid nematodes in the genus Mansonella: M. ozzardi, M. perstans, and M. streptocerca.
Life Cycle
Mansonella ozzardi
Geographic Distribution
Mansonella streptocerca is found in Africa; Mansonella perstans occurs in both Africa and South America; and Mansonella ozzardi occurs only ins the Americas, from Mexico south to South America and in the Caribbean.
Clinical Presentation
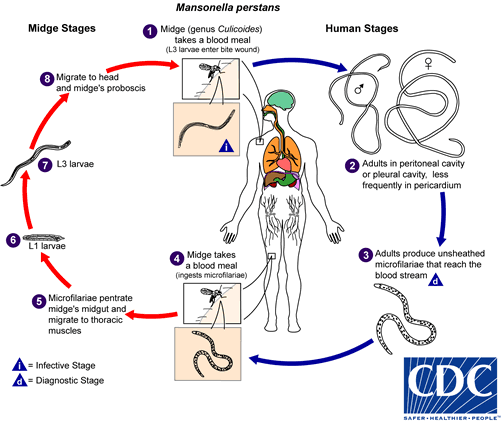
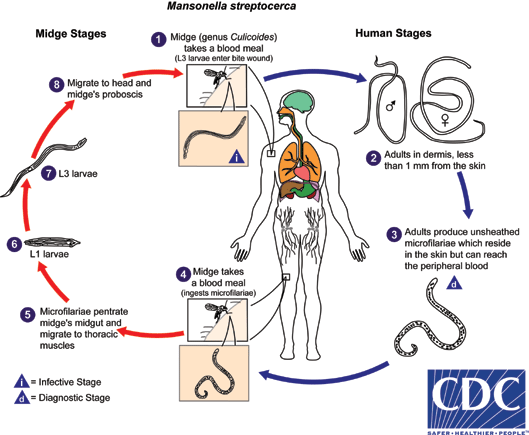
Infections by Mansonella perstans, while often asymptomatic, can be associated with angioedema, pruritus, fever, headaches, arthralgias, and neurologic manifestations. Mansonella streptocerca can cause skin manifestations including pruritus, papular eruptions and pigmentation changes. Eosinophilia is often prominent in filarial infections. Mansonella ozzardi can cause symptoms that include arthralgias, headaches, fever, pulmonary symptoms, adenopathy, hepatomegaly, and pruritus.
Microfilariae of Mansonella perstans.
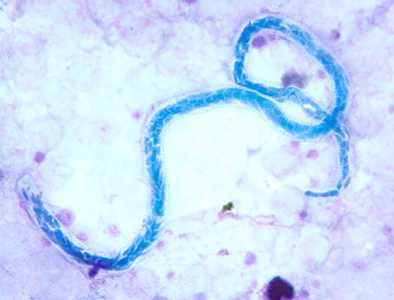
Figure A: Microfilaria of M. perstans in a thick blood smear stained with Giemsa, from a patient from Cameroon.
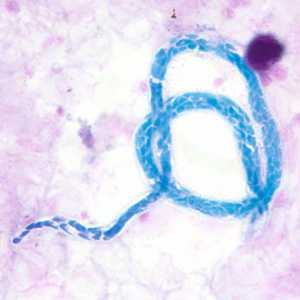
Figure B: Microfilaria of M. perstans in a thick blood smear stained with Giemsa, from a patient from Cameroon.
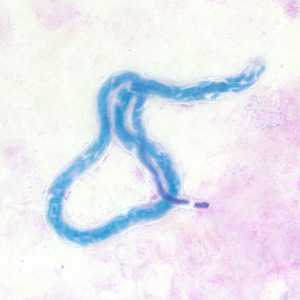
Figure C: Microfilaria of M. perstans in a thick blood smear stained with Giemsa, from a patient from Cameroon.
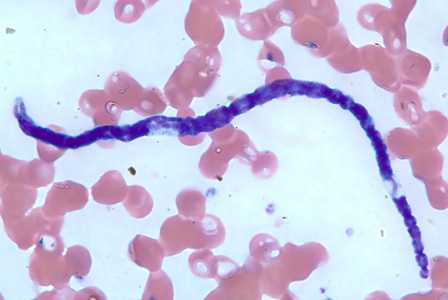
Figure D: Microfilaria of M. perstans in a thin blood smear from the same specimen as Figures A-C.
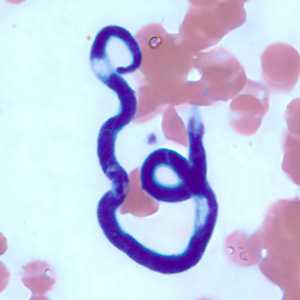
Figure E: Microfilaria of M. perstans in a thin blood smear from the same specimen as Figures A-D.
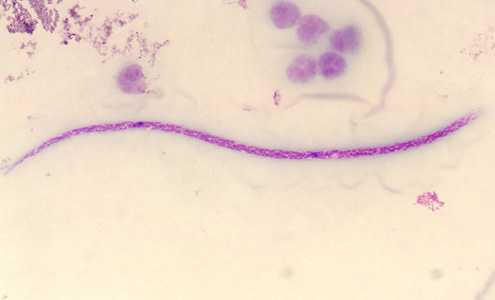
Figure F: Microfilaria of M. perstans in a thick blood smear stained with Giemsa. Image courtesy of the Parasitology Department, Public Health Lab, Ontario Agency for Health Protection and Promotion, Canada.
Microfilariae of M. ozzardi.
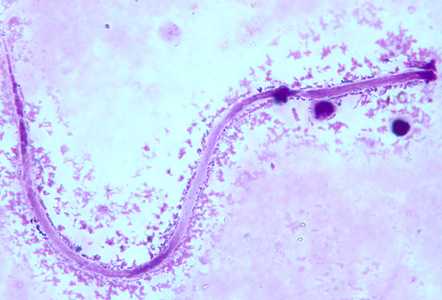
Figure A: Microfilaria of M. ozzardi in a thick blood smear, stained with Giemsa.
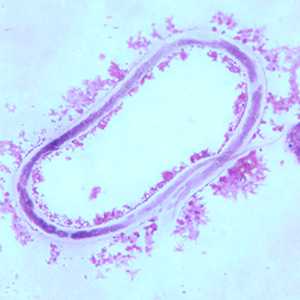
Figure B: Microfilaria of M. ozzardi in a thick blood smear, stained with Giemsa.
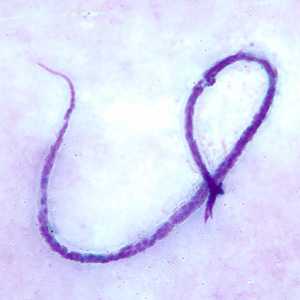
Figure C: Microfilaria of M. ozzardi in a thick blood smears, stained with Giemsa.
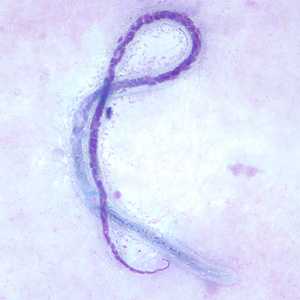
Figure D: Microfilaria of M. ozzardi in a thick blood smear, stained with Giemsa. Note the hook-like end to the tail in this figure.
Microfilariae of M. streptocerca.
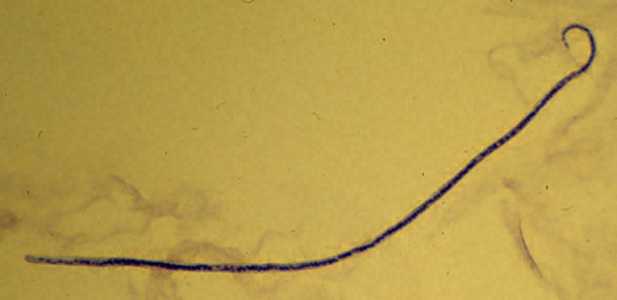
Figure A: Microfilaria of M. streptocerca, fixed in 2% formalin and stained with hematoxylin.
Laboratory Diagnosis
Mansonella perstans and M. ozzardi are usually diagnosed by the finding of microfilariae circulating in blood. Neither species exhibits periodicity. Mansonella streptocerca is usually diagnosed by finding microfilariae in skin snips.
Skin snips should be think enough to include the outer part of the dermal palpillae but not so thick as to produce bleeding. Skin snips should be placed immediately in normal saline or distilled water, just enough to cover the specimen. Microfilariae tend to emerge more rapidly in saline, however in either medium the microfilariae typically emerge in 30-60 min and can be seen in wet mount preparations. For a definitive diagnosis, allow the wet mount to dry, fix in methanol, and stain with Giemsa or hematoxylin-and-eosin.
Treatment Information
Treatment of Mansonella infections is poorly studied and thus recommendations are based on limited data.
Mansonella perstans
Treatment may vary based on regional strain differences, as Wolbachia were identified in M. perstans in Mali but not in Gabon or Uganda. Strains with Wolbachia should be treated with 6 weeks of doxycycline, which resulted in clearance of microfilaria in 97% of individuals at 12 months and 75% at 36 months. For strains that do not contain Wolbachia, treatment with 21 days of diethylcarbamazine in combination with 21 days of mebendazole resulted in clearance of microfilaria in 37% at one month. A 28-day course of mebendazole resulted in clearance of microfilaria in 21.7% at 1 month. Multiple treatments may be required for cure in individual patients, though there is not published evidence to guide a strategy. Neither ivermectin nor albendazole appear to have notable effects on microfilaremia.
Mansonella streptocerca
A community-based study of ivermectin found that a single dose of ivermectin suppresses microfilaria for a year or more. One year after a single dose, 46% of individuals had no detectable microfilaria on skin biopsy.
Mansonella ozzardi
Case reports suggest that single dose ivermectin might be a useful treatment of M. ozzardi infections. There is also evidence that M. ozzardi contain Wolbachia, so doxycycline might be an effective treatment; however there are no published data to support this treatment option. Diethylcarbamazine has been shown to have no effect on M. ozzardi.
Doxycycline
Doxycycline is available for human use in the United States.
Note on Treatment in Pregnancy
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir
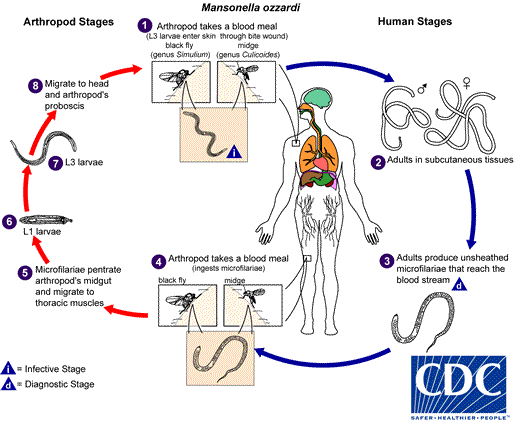
 . They develop into adults that commonly reside in subcutaneous tissues
. They develop into adults that commonly reside in subcutaneous tissues  . Adult worms are rarely found in humans. The size range for females worms is 65 to 81 mm in length and 0.21 to 0.25 mm in diameter but unknown for males. Adults worms recovered from experimentally infected Patas monkeys measured 24 to 28 mm in length and 70 to 80 µm in diameter (males) and 32 to 62 mm in length and .130 to .160 mm in diameter (females). Adults produce unsheathed and non-periodic microfilariae that reach the blood stream
. Adult worms are rarely found in humans. The size range for females worms is 65 to 81 mm in length and 0.21 to 0.25 mm in diameter but unknown for males. Adults worms recovered from experimentally infected Patas monkeys measured 24 to 28 mm in length and 70 to 80 µm in diameter (males) and 32 to 62 mm in length and .130 to .160 mm in diameter (females). Adults produce unsheathed and non-periodic microfilariae that reach the blood stream  . The arthropod ingests microfilariae during a blood meal
. The arthropod ingests microfilariae during a blood meal  . After ingestion, the microfilariae migrate from the arthropod's midgut through the hemocoel to the thoracic muscles
. After ingestion, the microfilariae migrate from the arthropod's midgut through the hemocoel to the thoracic muscles  . There the microfilariae develop into first-stage larvae
. There the microfilariae develop into first-stage larvae  and subsequently into third-stage infective larvae
and subsequently into third-stage infective larvae  . The third-stage infective larvae migrate to arthropod's proboscis
. The third-stage infective larvae migrate to arthropod's proboscis  and can infect another human when the arthropod takes a blood meal
and can infect another human when the arthropod takes a blood meal