Lymphatic Filariasis
[Brugia malayi] [Brugia timori] [Wuchereria bancrofti]
Causal Agent
The filarid nematodes Wuchereria bancrofti, Brugia malayi, and (less-commonly) B. timori. Humans can also be infected with several zoonotic Brugia species.
Brugia Malayi
Wuchereria bancrofti
Geographic Distribution
Among the agents of lymphatic filariasis, Wuchereria bancrofti is encountered in tropical areas worldwide; Brugia malayi is limited to Asia; and Brugia timori is restricted to some islands of Indonesia.
Clinical Presentation
Lymphatic filariasis most often consists of asymptomatic microfilaremia. Some patients develop lymphatic dysfunction causing lymphedema and elephantiasis (frequently in the lower extremities) and, with Wuchereria bancrofti, hydrocele and scrotal elephantiasis. Episodes of febrile lymphangitis and lymphadenitis may occur. Persons who have newly arrived in disease-endemic areas can develop afebrile episodes of lymphangitis and lymphadenitis. An additional manifestation of filarial infection, mostly in Asia, is pulmonary tropical eosinophilia syndrome, with nocturnal cough and wheezing, fever, and eosinophilia.
Microfilariae of Wuchereria bancrofti.
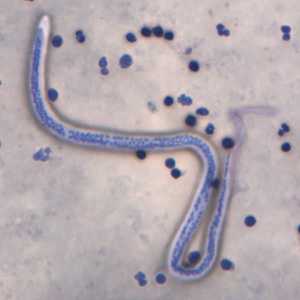
Figure A: Microfilaria of W. bancrofti in a thick blood smear stained with Giemsa. Image courtesy of the Oregon State Public Health Laboratory.
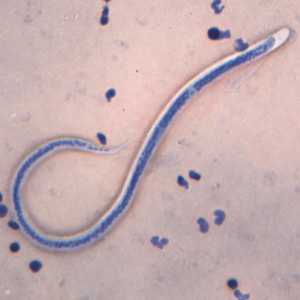
Figure B: Microfilaria of W. bancrofti in a thick blood smear stained with Giemsa. Image courtesy of the Oregon State Public Health Laboratory.
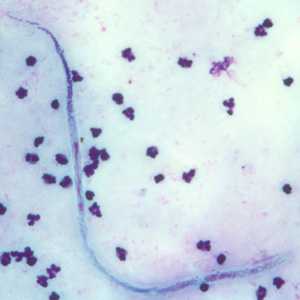
Figure C: Microfilaria of W. bancrofti in a thick blood smear, stained with Giemsa.
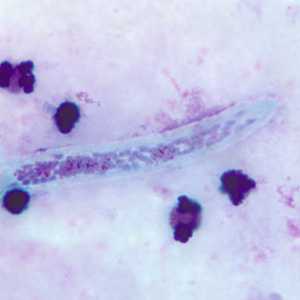
Figure D: Close-up of the anterior end of the worm in Figure C.
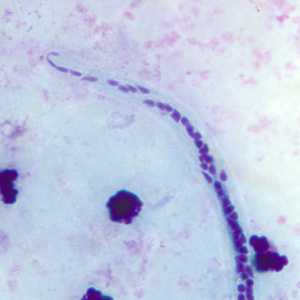
Figure E: Close-up of the posterior end of the worm in Figure C.
Adults of W. bancrofti.
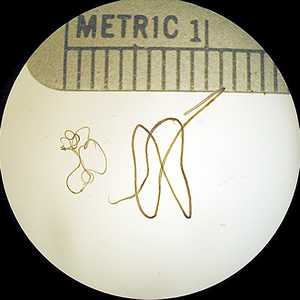
Figure A: Adults of W. bancrofti. The male worm is on the left; the female is on the right.
Microfilariae of Brugia malayi.
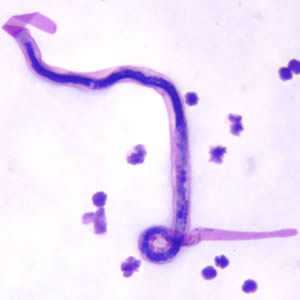
Figure A: Microfilaria of B. malayi in a thick blood smear, stained with Giemsa.
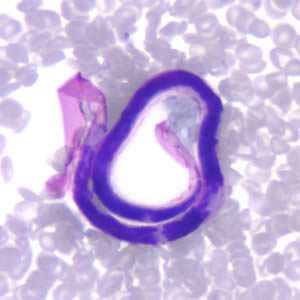
Figure B: Microfilaria of B. malayi in a thin blood smear, stained with Giemsa.
Microfilariae of B. timori.
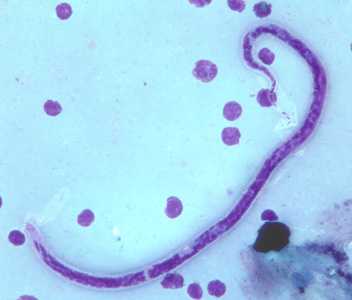
Figure A: Microfilaria of B. timori in a thick blood smear from a patient from Indonesia, stained with Giemsa and captured at 500x oil magnification. Image from a specimen courtesy of Dr. Thomas C. Orihel, Tulane University, New Orleans, LA.
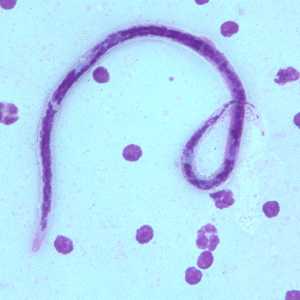
Figure B: Microfilaria of B. timori in a thick blood smear from a patient from Indonesia, stained with Giemsa and captured at 500x oil magnification. Image from a specimen courtesy of Dr. Thomas C. Orihel, Tulane University, New Orleans, LA.
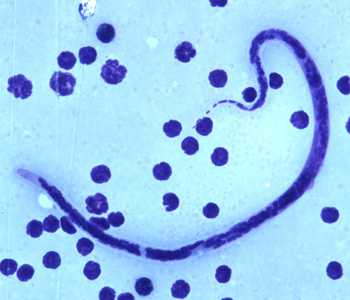
Figure C: Microfilaria of B. timori in a thick blood smear from a patient from Indonesia, stained with Giemsa and captured at 500x oil magnification. Image from a specimen courtesy of Dr. Thomas C. Orihel, Tulane University, New Orleans, LA.
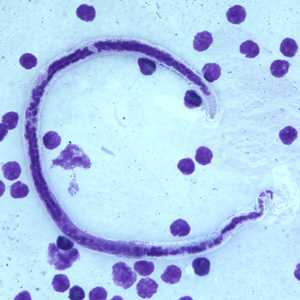
Figure D: Microfilaria of B. timori in a thick blood smear from a patient from Indonesia, stained with Giemsa and captured at 500x oil magnification. Image from a specimen courtesy of Dr. Thomas C. Orihel, Tulane University, New Orleans, LA.
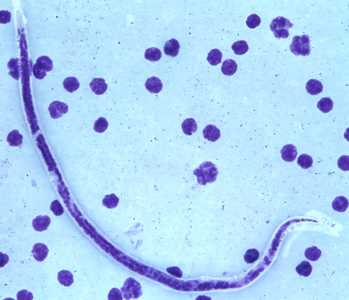
Figure E: Microfilaria of B. timori in a thick blood smear from a patient from Indonesia, stained with Giemsa and captured at 500x oil magnification. Image from a specimen courtesy of Dr. Thomas C. Orihel, Tulane University, New Orleans, LA.
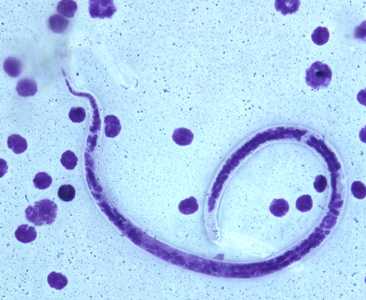
Figure F: Microfilaria of B. timori in a thick blood smear from a patient from Indonesia, stained with Giemsa and captured at 500x oil magnification. Image from a specimen courtesy of Dr. Thomas C. Orihel, Tulane University, New Orleans, LA.
Adults of Brugia spp. in tissue.
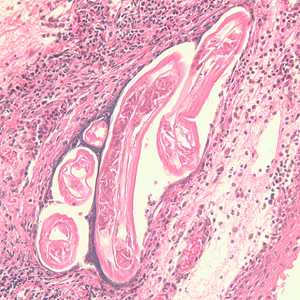
Figure A: Section of an adult of Brugia sp. from a lymph node, stained with hematoxylin and eosin (H&E). Image taken at 200x magnification.
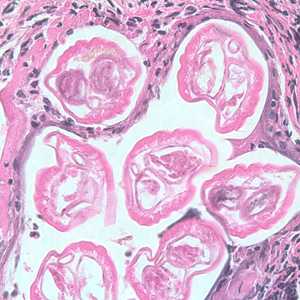
Figure B: Section of an adult of Brugia sp. from a lymph node, stained with hematoxylin and eosin (H&E). Image taken at 400x magnification.
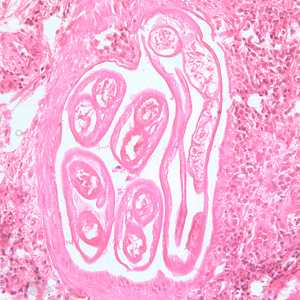
Figure C: Section of an adult of Brugia sp. from a femoral lymph node, stained with H&E. Image taken at 200x magnification.
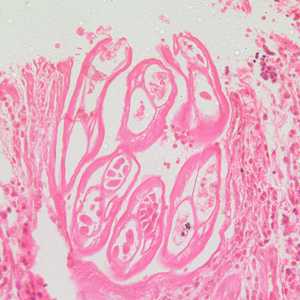
Figure D: Section of an adult of Brugia sp. from a femoral lymph node, stained with H&E. Image taken at 200x magnification.
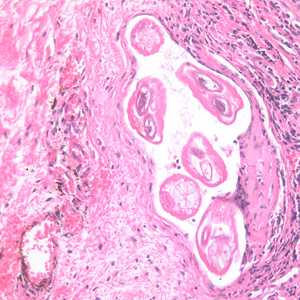
Figure E: Section of an adult of Brugia sp. from the conjunctiva of a patient from Ecuador, stained with H&E. Image taken at 200x magnification.
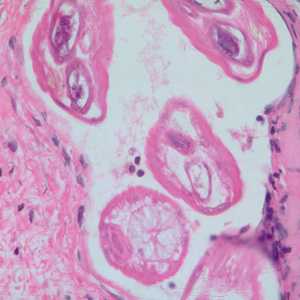
Figure F: Section of an adult of Brugia sp. from the conjunctiva of a patient from Ecuador, stained with H&E. Image taken at 500x oil magnification.
Diagnostic Findings
Microscopy
Lymphatic filariasis is usually identified by the finding of microfilaria in peripheral blood smears (thick or thin) stained with Giemsa or hematoxylin-and-eosin. For increased sensitivity, concentration techniques can be used. These include centrifugation of the blood sample lysed in 2% formalin (Knott's technique), or filtration through a Nucleopore® membrane. Microfilariae of Wuchereria and Brugia exhibit a nocturnal periodicity and an accurate diagnosis is best achieved on smears collected at night (10 PM-2 AM). Adults may be identified in biopsy specimens of lymphatic tissue.
Antigen Detection
Antigen detection using an immunoassay for circulating filarial antigens constitutes a useful diagnostic approach because sensitivity for detection of microfilariae can be low and variable. Unlike microfilariae with nocturnal periodicity, filarial antigens can be detected in blood samples collected at any time of day. A rapid format immunochromatographic test has been shown to be a useful and sensitive tool for the detection of Wuchereria bancrofti antigen and is being used widely by lymphatic filariasis elimination programs. Currently, this test is not licensed for use in the United States and cannot be used for patient diagnosis.
Treatment Information
The main goal of treatment of an infected person is to kill the adult worm. Diethylcarbamazine citrate (DEC), which is both microfilaricidal and active against the adult worm, is the drug of choice for lymphatic filariasis. The late phase of chronic disease is not affected by chemotherapy. Ivermectin is effective against the microfilariae of W. bancrofti, but has no effect on the adult parasite.
Because lymphatic filariasis is rare in the U.S., DEC is no longer approved by the Food and Drug Administration (FDA) and cannot be sold in the U.S. Physicians can obtain the medication from CDC after confirmed positive lab results. Call: 404-718-4745. Treatment of lymphatic filariasis in adults and children > 18 months of age involves either a 1 day or 12 day treatment course (6mg/kg/day). One day treatment is generally as effective as the 12-day regimen. For tropical pulmonary eosinophilia (TPE), a longer DEC treatment course of 14-21 days is generally recommended. DEC is generally well tolerated. Side effects are generally limited and depend on the number of microfilariae in the blood. The most common side effects are dizziness, nausea, fever, headache, or pain in muscles or joints.
DEC is contraindicated in patients who may also have onchocerciasis. Prior to DEC treatment for lymphatic filariasis, onchocerciasis should be excluded in all patients with a consistent exposure history due to the possibility of severe exacerbations of skin and eye involvement (Mazzotti reaction). In addition, DEC should be used with extreme caution in patients with circulating Loa loa microfilarial levels > 2,500/mm3 due to the potential for life-threatening side effects, including encephalopathy and renal failure. Neither steroids pre-treatment nor slow dose escalation prevents these complications. Consultation with a tropical medicine specialist is recommended in these scenarios.
The drug ivermectin kills only the microfilariae, but not the adult worm; the adult worm is responsible for the pathology of lymphedema and hydrocele.
Some studies have shown adult worm killing with treatment with doxycycline (200mg/day for 4–6 weeks).
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir
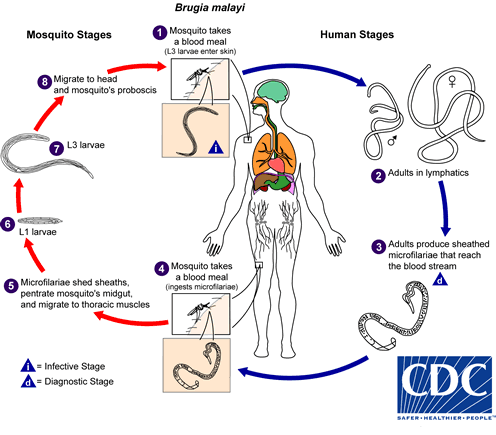
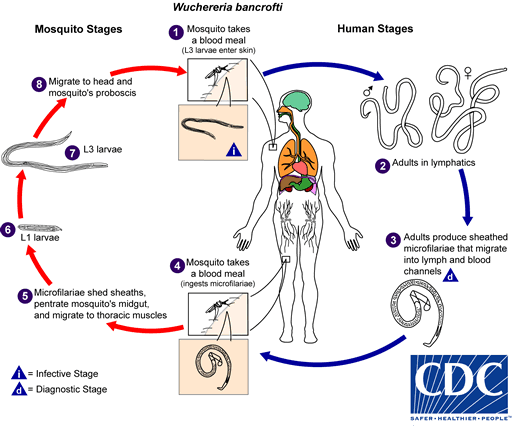
 . They develop into adults that commonly reside in the lymphatics
. They develop into adults that commonly reside in the lymphatics  . The adult worms resemble those of Wuchereria bancrofti but are smaller. Female worms measure 43 to 55 mm in length by 130 to 170 μm in width, and males measure 13 to 23 mm in length by 70 to 80 μm in width. Adults produce microfilariae, measuring 177 to 230 μm in length and 5 to 7 μm in width, which are sheathed and have nocturnal periodicity. The microfilariae migrate into lymph and enter the blood stream reaching the peripheral blood
. The adult worms resemble those of Wuchereria bancrofti but are smaller. Female worms measure 43 to 55 mm in length by 130 to 170 μm in width, and males measure 13 to 23 mm in length by 70 to 80 μm in width. Adults produce microfilariae, measuring 177 to 230 μm in length and 5 to 7 μm in width, which are sheathed and have nocturnal periodicity. The microfilariae migrate into lymph and enter the blood stream reaching the peripheral blood  . A mosquito ingests the microfilariae during a blood meal
. A mosquito ingests the microfilariae during a blood meal  . After ingestion, the microfilariae lose their sheaths and work their way through the wall of the proventriculus and cardiac portion of the midgut to reach the thoracic muscles
. After ingestion, the microfilariae lose their sheaths and work their way through the wall of the proventriculus and cardiac portion of the midgut to reach the thoracic muscles  . There the microfilariae develop into first-stage larvae
. There the microfilariae develop into first-stage larvae  and subsequently into third-stage larvae
and subsequently into third-stage larvae  . The third-stage larvae migrate through the hemocoel to the mosquito's proboscis
. The third-stage larvae migrate through the hemocoel to the mosquito's proboscis  and can infect another human when the mosquito takes a blood meal
and can infect another human when the mosquito takes a blood meal