
Intestinal Capillariasis
[Capillaria philippinensis]
Causal Agent
The nematode (roundworm) Capillaria philippinensis causes human intestinal capillariasis.
Life Cycle
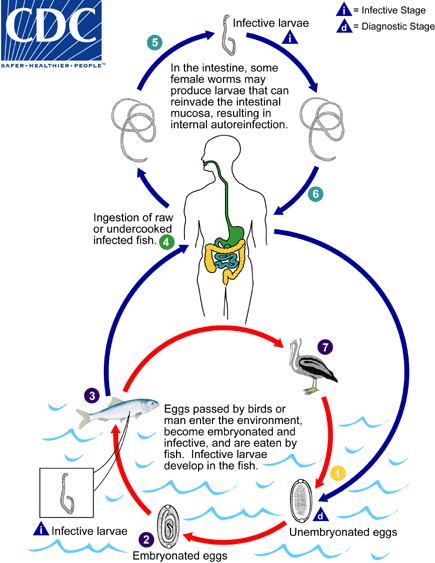
Typically,unembryonated eggs are passed in the human stool  and become embryonated in the external environment
and become embryonated in the external environment  ; after ingestion by freshwater fish, larvae hatch, penetrate theintestine, and migrate to the tissues
; after ingestion by freshwater fish, larvae hatch, penetrate theintestine, and migrate to the tissues  . Ingestion of raw or undercooked fish results in infection of thehuman host. Humans are the only demonstrated hosts
. Ingestion of raw or undercooked fish results in infection of thehuman host. Humans are the only demonstrated hosts  . The adults of Capillaria philippinensis (males: 2.3 to 3.2mm; females: 2.5 to 4.3 mm) reside in the human small intestine, where theyburrow in the mucosa
. The adults of Capillaria philippinensis (males: 2.3 to 3.2mm; females: 2.5 to 4.3 mm) reside in the human small intestine, where theyburrow in the mucosa  . The females deposit unembryonated eggs. Some of thesebecome embryonated in the intestine, and release larvae that can causeautoinfection. This leads to hyperinfection (a massive number of adult worms)
. The females deposit unembryonated eggs. Some of thesebecome embryonated in the intestine, and release larvae that can causeautoinfection. This leads to hyperinfection (a massive number of adult worms)  . Capillaria philippinesis is currently considered aparasite of fish eating birds, which seem to be the natural definitive host
. Capillaria philippinesis is currently considered aparasite of fish eating birds, which seem to be the natural definitive host  .
.
Geographic Distribution
Capillaria philippinensis is endemic in the Philippines and also occurs in Thailand. Rare cases have been reported from other Asian countries, the Middle East, and Colombia.
Clinical Presentation
Intestinal capillariasis (caused by C. philippinensis) manifests as abdominal pain and diarrhea, which, if untreated, may become severe because of autoinfection. A protein-losing enteropathy can develop which may result in cachexia and death.
Capillaria philippinensis eggs.
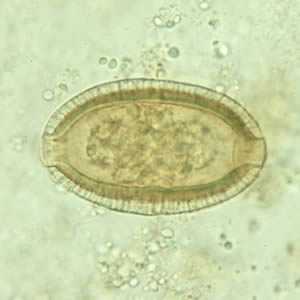
Figure A: Egg of C. philippinensis in an unstained wet mount of stool.
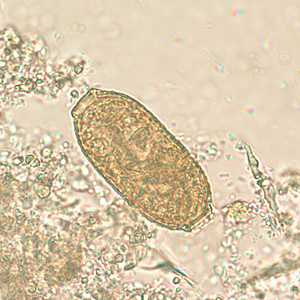
Figure B: Egg of C. philippinensis in an unstained wet mount of stool.
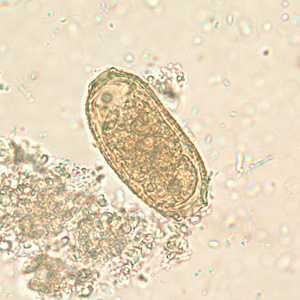
Figure C: Egg of C. philippinensis in an unstained wet mount of stool.
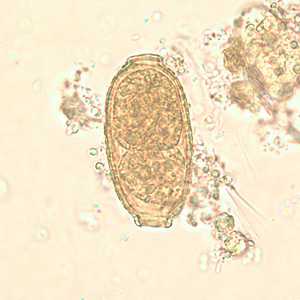
Figure D: Egg of C. philippinensis in an unstained wet mount of stool.
Capillaria philippinensis adults.
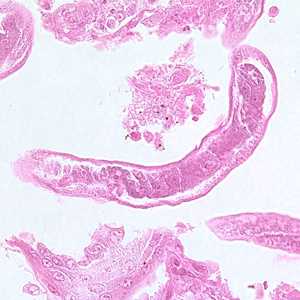
Figure A: Longitudinal section of an adult of C. philippinensis from an intestinal biopsy specimen stained with hematoxylin and eosin (H&E).
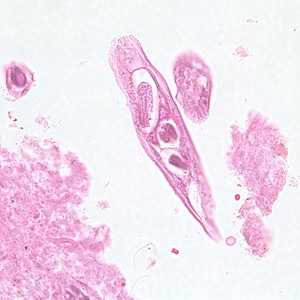
Figure B: Longitudinal section of an adult of C. philippinensis from an intestinal biopsy specimen stained with hematoxylin and eosin (H&E).
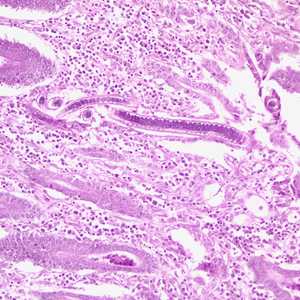
Figure C: Longitudinal section of an adult C. philippinensis from an intestinal biopsy specimen, stained with H&E.
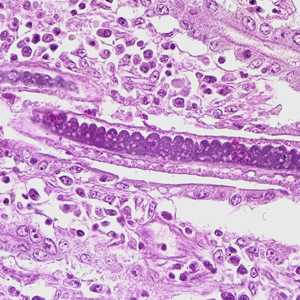
Figure D: Higher magnification of Figure C, showing stichocytes within the adult worm.
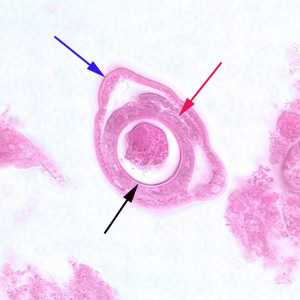
Figure E: Cross-section of a gravid adult female C. philippinensis from an intestinal biopsy specimen, stained with H&E. Shown in this figure are a bacillary band (blue arrow), the intestine (red arrow) and uterus containing an egg in cross-section (black arrow).
Diagnostic Findings
The specific diagnosis of C. philippinensis is established by finding eggs, larvae and/or adult worms in the stool, or in intestinal biopsies. Unembryonated eggs are the typical stage found in the feces. In severe infections, embryonated eggs, larvae, and even adult worms can be found in the feces.
More on: Morphologic comparison with other intestinal parasites
Treatment Information
Mebendazole*, is the drug of choice for adults, 200 mg orally twice a day for 20 days; the pediatric dosage is the same.
Alternative:
Albendazole*, adults, 400 mg orally once a day for 10 days; the pediatric dosage is the same.
(Note: Albendazole must be taken with food; a fatty meal increases oral bioavailability.)
Mebendazole
Mebendazole is available in the United States only through compounding pharmacies.
Note on Treatment in Pregnancy
Albendazole
Oral albendazole is available for human use in the United States.
Note on Treatment in Pregnancy
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir