
Heterophyiasis
[Heterophyes heterophyes]
Causal Agents
The trematode Heterophyes heterophyes, a minute intestinal fluke.
Life Cycle
Adults release embryonated eggs each with a fully-developed miracidium, and eggs are passed in the host's feces . After ingestion by a suitable snail (first intermediate host), the eggs hatch and release miracidia which penetrate the snail’s intestine
. After ingestion by a suitable snail (first intermediate host), the eggs hatch and release miracidia which penetrate the snail’s intestine . Genera Cerithidia and Pironella are important snail hosts in Asia and the Middle East respectively. The miracidia undergo several developmental stages in the snail, i.e. sporocysts
. Genera Cerithidia and Pironella are important snail hosts in Asia and the Middle East respectively. The miracidia undergo several developmental stages in the snail, i.e. sporocysts , rediae
, rediae , and cercariae
, and cercariae . Many cercariae are produced from each redia. The cercariae are released from the snail
. Many cercariae are produced from each redia. The cercariae are released from the snail and encyst as metacercariae in the tissues of a suitable fresh/brackish water fish (second intermediate host)
and encyst as metacercariae in the tissues of a suitable fresh/brackish water fish (second intermediate host) . The definitive host becomes infected by ingesting undercooked or salted fish containing metacercariae
. The definitive host becomes infected by ingesting undercooked or salted fish containing metacercariae . After ingestion, the metacercariae excyst, attach to the mucosa of the small intestine
. After ingestion, the metacercariae excyst, attach to the mucosa of the small intestine and mature into adults (measuring 1.0 to 1.7 mm by 0.3 to 0.4 mm)
and mature into adults (measuring 1.0 to 1.7 mm by 0.3 to 0.4 mm) . In addition to humans, various fish-eating mammals (e.g., cats and dogs) and birds can be infected by Heterophyes heterophyes
. In addition to humans, various fish-eating mammals (e.g., cats and dogs) and birds can be infected by Heterophyes heterophyes  .
.
Geographic Distribution
Egypt, the Middle East, and Far East.
Clinical Presentation
The main symptoms are diarrhea and colicky abdominal pain. Migration of the eggs to the heart, resulting in potentially fatal myocardial and valvular damage, has been reported from the Philippines. Migration to other organs (e.g., brain) has also been reported.
Adult of Heterophyes heterophyes.
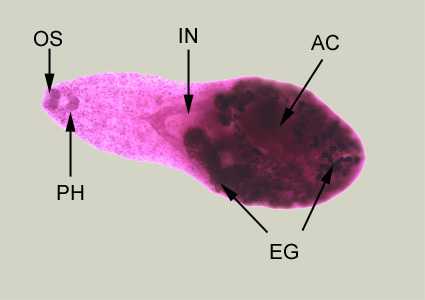
Figure A: Adult of H. heterophyes, stained with carmine. In this figure, the following structures are labeled: oral sucker (OS), pharynx (PH), intestine (IN), ventral sucker, or acetabulum (AC), and eggs within the uterus (UT)
Snail intermediate hosts of Heterophyes heterophyes.

Figure A: Cerithideopsilla cingulata, an intermediate host for H. heterophyes in southeast Asia. Image courtesy of Conchology, Inc, Mactan Island, Philippines
Laboratory Diagnosis
The diagnosis is based on the microscopic identification of eggs in the stool. However, the eggs are indistinguishable from those of Metagonimus yokogawai and resemble those of Clonorchis and Opisthorchis.
Treatment Information
Praziquantel* is the drug of choice.
Praziquantel
Oral praziquantel is available for human use in the United States.
Note on Treatment in Pregnancy
* This drug is approved by the FDA, but considered investigational for this purpose.
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir