
Hepatic Capillariasis
[Capillaria hepatica]
Causal Agent
The nematode (roundworm) Capillaria hepatica causes hepatic capillariasis in humans.
Life Cycle
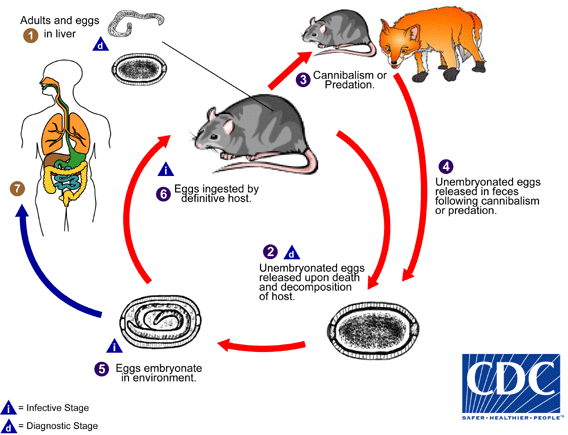
Capillaria hepatica has a direct life cycle that requires only one host. Adult worms invade the liver of the host (usually rodents, but may also be pigs, carnivores and primates, including humans), and lay hundreds of eggs in the surrounding parenchyma ![]() . The eggs are not passed in the feces of the host, and remain in the liver until the animal dies and decomposes
. The eggs are not passed in the feces of the host, and remain in the liver until the animal dies and decomposes ![]() , or is eaten by a predator or scavenger
, or is eaten by a predator or scavenger ![]() . Eggs ingested by such an animal are unembryonated, are not infectious, and are passed in the feces, providing an efficient mechanism to release eggs into the environment
. Eggs ingested by such an animal are unembryonated, are not infectious, and are passed in the feces, providing an efficient mechanism to release eggs into the environment ![]() . Cannibalism has been reported as an important role in transmission among rodent populations. Eggs embryonate in the environment
. Cannibalism has been reported as an important role in transmission among rodent populations. Eggs embryonate in the environment ![]() , where they require air and damp soil to become infective. Under optimal conditions, this takes about 30 days. The cycle continues when embryonated eggs are eaten by a suitable mammalian host
, where they require air and damp soil to become infective. Under optimal conditions, this takes about 30 days. The cycle continues when embryonated eggs are eaten by a suitable mammalian host ![]() . Infective eggs hatch in the intestine, releasing larvae. The larvae migrate via the portal vein to the liver. Larvae take about four weeks to mature into adults and mate. Humans are usually infected after ingesting embryonated eggs in fecal-contaminated food, water, or soil
. Infective eggs hatch in the intestine, releasing larvae. The larvae migrate via the portal vein to the liver. Larvae take about four weeks to mature into adults and mate. Humans are usually infected after ingesting embryonated eggs in fecal-contaminated food, water, or soil ![]() . Occasionally in humans, larvae will migrate to the lungs, kidneys, or other organs. The presence of C. hepatica eggs in human stool during routine ova-and-parasite (O&P) examinations indicates spurious passage of ingested eggs, and not a true infection. Diagnosis in humans is usually achieved by finding adults and eggs in biopsy or autopsy specimens.
. Occasionally in humans, larvae will migrate to the lungs, kidneys, or other organs. The presence of C. hepatica eggs in human stool during routine ova-and-parasite (O&P) examinations indicates spurious passage of ingested eggs, and not a true infection. Diagnosis in humans is usually achieved by finding adults and eggs in biopsy or autopsy specimens.
Geographic Distribution
Rare cases of human infections with C. hepatica have been reported worldwide.
Clinical Presentation
Hepatic capillariasis (C. hepatica) manifests as an acute or subacute hepatitis with eosinophilia, with possible dissemination to other organs. It may be fatal.
Capillaria hepatica eggs.
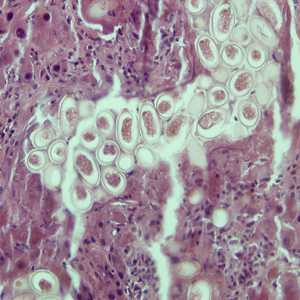
Figure A: Eggs of C. hepatica in liver stained with hematoxylin and eosin (H&E).

Figure A: Eggs of C. hepatica in liver stained with hematoxylin and eosin (H&E).
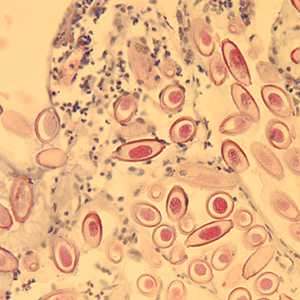
Figure D: Eggs of C. hepatica in liver stained with H&E.
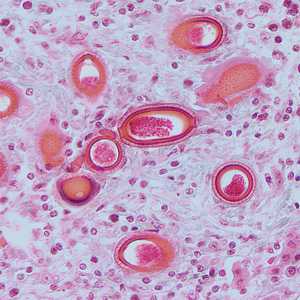
Figure C: Eggs of C. hepatica in liver stained with H&E.
Capillaria hepatica adults.
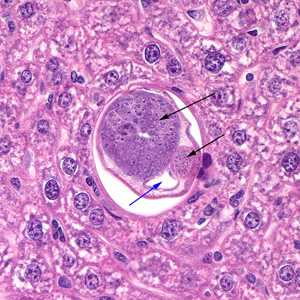
Figure A: Cross section of a male C. hepatica in liver tissue, stained with hematoxylin and eosin (H&E). Note the presence of the intestine (blue arrow) and the coiled sections of the testes (black arrows).
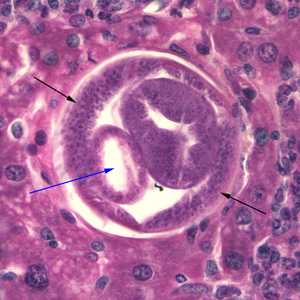
Figure B: Cross section of C. hepatica in liver tissue, stained with H&E. Note the presence of the intestine (blue arrow) and bacillary bands (black arrows).
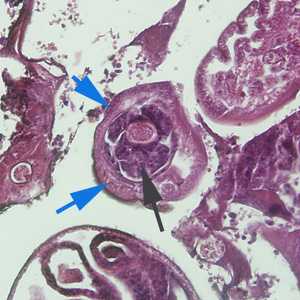
Figure C: Cross-section of C. hepatica in liver tissue, stained with H&E. Note the presence of a stichocyte (black arrow) and bacillary bands (blue arrows). Image taken at 200x magnification.
Diagnostic Findings
The specific diagnosis of C. hepatica infection is based on demonstrating the adult worms and/or eggs in liver tissue at biopsy or necropsy. (Note: identification of C. hepatica eggs in the stool is a spurious finding, which does not result from infection of the human host, but from ingestion by that host of livers from infected animals.)
More on: Morphologic comparison with other intestinal parasites
Treatment Information
Mebendazole*, is the drug of choice for adults, 200 mg orally twice a day for 20 days; the pediatric dosage is the same.
Alternative:
Albendazole*, adults, 400 mg orally once a day for 10 days; the pediatric dosage is the same.
(Note: Albendazole must be taken with food; a fatty meal increases oral bioavailability.)
Mebendazole
Mebendazole is available in the United States only through compounding pharmacies.
Note on Treatment in Pregnancy
Albendazole
Oral albendazole is available for human use in the United States.
Note on Treatment in Pregnancy
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir