
Diphyllobothriasis
[Diphyllobothrium latum] [Diphyllobothrium spp.]
Causal Agents
The cestode Diphyllobothrium latum (the fish or broad tapeworm), the largest human tapeworm. Several other Diphyllobothrium species have been reported to infect humans, but less frequently; they include D. pacificum, D. cordatum, D. ursi, D. dendriticum, D. lanceolatum, D. dalliae, and D. yonagoensis.
Life Cycle
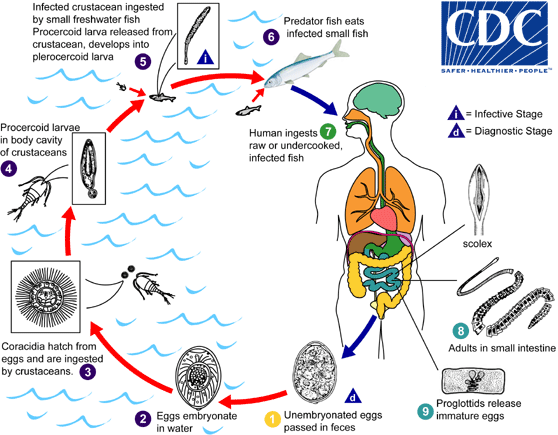
Immature eggs are passed in feces . Under appropriate conditions, the eggs mature (approximately 18 to 20 days)
. Under appropriate conditions, the eggs mature (approximately 18 to 20 days) and yield oncospheres which develop into a coracidia
and yield oncospheres which develop into a coracidia . After ingestion by a suitable freshwater crustacean (the copepod first intermediate host) the coracidia develop into procercoid larvae
. After ingestion by a suitable freshwater crustacean (the copepod first intermediate host) the coracidia develop into procercoid larvae . Following ingestion of the copepod by a suitable second intermediate host, typically minnows and other small freshwater fish, the procercoid larvae are released from the crustacean and migrate into the fish flesh where they develop into a plerocercoid larvae (sparganum)
. Following ingestion of the copepod by a suitable second intermediate host, typically minnows and other small freshwater fish, the procercoid larvae are released from the crustacean and migrate into the fish flesh where they develop into a plerocercoid larvae (sparganum) . The plerocercoid larvae are the infective stage for humans. Because humans do not generally eat undercooked minnows and similar small freshwater fish, these do not represent an important source of infection. Nevertheless, these small second intermediate hosts can be eaten by larger predator species, e.g., trout, perch, walleyed pike
. The plerocercoid larvae are the infective stage for humans. Because humans do not generally eat undercooked minnows and similar small freshwater fish, these do not represent an important source of infection. Nevertheless, these small second intermediate hosts can be eaten by larger predator species, e.g., trout, perch, walleyed pike . In this case, the sparganum can migrate to the musculature of the larger predator fish and humans can acquire the disease by eating these later intermediate infected host fish raw or undercooked
. In this case, the sparganum can migrate to the musculature of the larger predator fish and humans can acquire the disease by eating these later intermediate infected host fish raw or undercooked . After ingestion of the infected fish, the plerocercoid develop into immature adults and then into mature adult tapeworms which will reside in the small intestine. The adults of D. latum attach to the intestinal mucosa by means of the two bilateral groves (bothria) of their scolex
. After ingestion of the infected fish, the plerocercoid develop into immature adults and then into mature adult tapeworms which will reside in the small intestine. The adults of D. latum attach to the intestinal mucosa by means of the two bilateral groves (bothria) of their scolex  . The adults can reach more than 10 m in length, with more than 3,000 proglottids. Immature eggs are discharged from the proglottids (up to 1,000,000 eggs per day per worm)
. The adults can reach more than 10 m in length, with more than 3,000 proglottids. Immature eggs are discharged from the proglottids (up to 1,000,000 eggs per day per worm) and are passed in the feces
and are passed in the feces . Eggs appear in the feces 5 to 6 weeks after infection. In addition to humans, many other mammals can also serve as definitive hosts for D. latum.
. Eggs appear in the feces 5 to 6 weeks after infection. In addition to humans, many other mammals can also serve as definitive hosts for D. latum.
Geographic Distribution
Diphyllobothriasis occurs in the Northern Hemisphere (Europe, North America, and Asia) and in South America (Uruguay and Chile). Freshwater fish infected with Diphyllobothrium sp. larva may be transported to and consumed in geographic areas where active transmission does not occur, resulting in human diphyllobothriasis. For example, cases of D. latum infection associated with consumption of imported fish have been reported in Brazil.
Clinical Presentation
Diphyllobothriasis can be a long-lasting infection (decades). Most infections are asymptomatic. Manifestations may include abdominal discomfort, diarrhea, vomiting, and weight loss. Vitamin B12 deficiency with pernicious anemia may occur. Massive infections may result in intestinal obstruction. Migration of proglottids can cause cholecystitis or cholangitis.
Diphyllobothrium latum eggs in wet mounts.
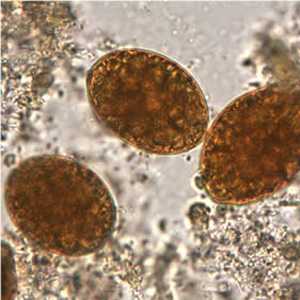
Figure A: Eggs of D. latum in an iodine-stained wet mount. Image courtesy of the Oregon State Public Health Laboratory.
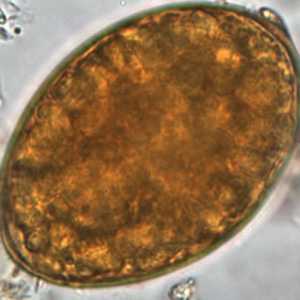
Figure B: Note the knob at the abopercular end. Image courtesy of the Oregon State Public Health Laboratory.
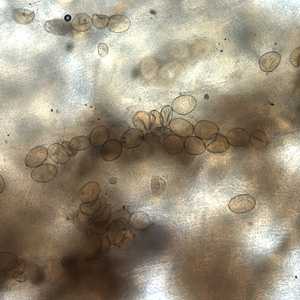
Figure C: Eggs of D. latum within a proglottid. Image courtesy of the Florida State Public Health Laboratory.
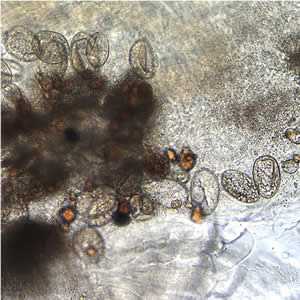
Figure D: Eggs of D. latum within a proglottid.
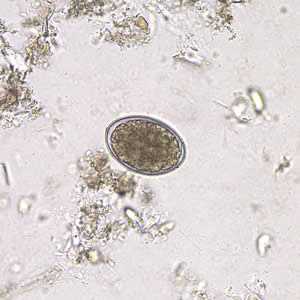
Figure E: Eggs of D. latum in an unstained wet mount.
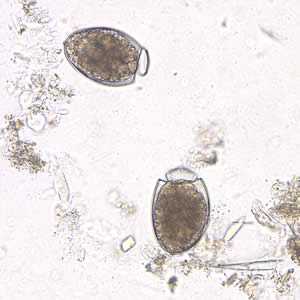
Figure F: Eggs of D. latum in an unstained wet mount of stool. Note the opercula are open.
Eggs of Diphyllobothrium spp. eggs in wet mounts.
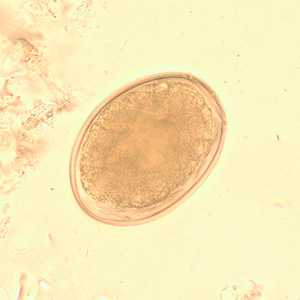
Figure A: Egg of Diphyllobothrium sp. in an unstained wet mount.
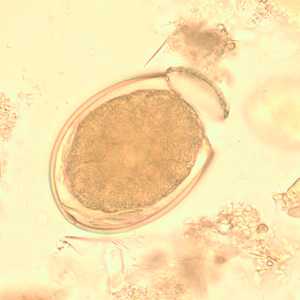
Figure B: Egg of D. latum in an unstained wet-mount of stool.
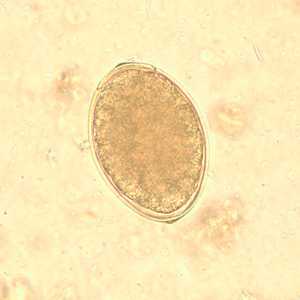
Figure C: Egg of Diphyllobothrium sp. in an unstained wet mount.
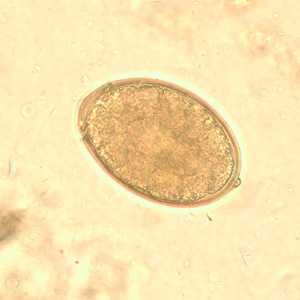
Figure D: Egg of Diphyllobothrium sp. in an unstained wet mount.
Proglottids of Diphyllobothrium spp.
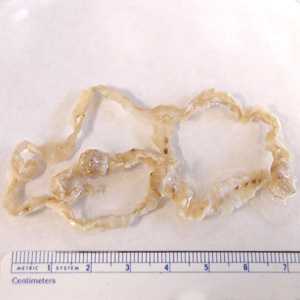
Figure A: Section of an adult D. latum containing many proglottids. The scolex was not present in this specimen. Image courtesy of the Florida State Public Health Laboratory.
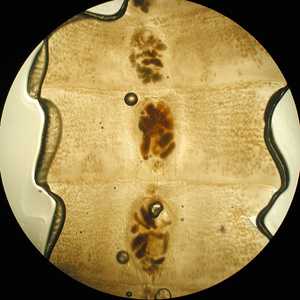
Figure B: Close-up of a few of the proglottids from the specimen in Figure A, showing the rosette-shaped uterus at the center of each proglottid.
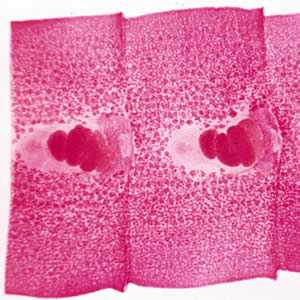
Figure C: Carmine-stained proglottids of D. latum, showing the rosette-shaped ovaries.
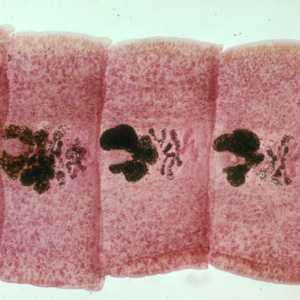
Figure D:Carmine-stained proglottids of D. latum, showing the rosette-shaped ovaries.
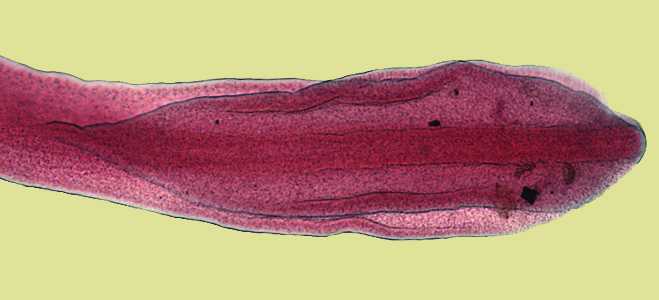
Figure E: Scolex of D. latum.
Diphyllobothrium adults and eggs in tissue, stained with hematoxylin and eosin (H&E).
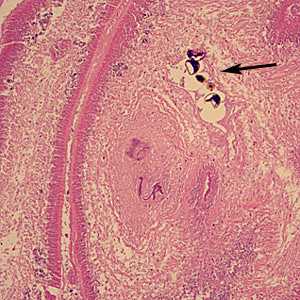
Figure A: Sections of gravid proglottids containing eggs (arrow) of Diphyllobothrium sp. in intestinal tissue, stained with H&E. Image taken at 100x magnification. Image courtesy of the University of Washington Medical Center, Seattle, WA.
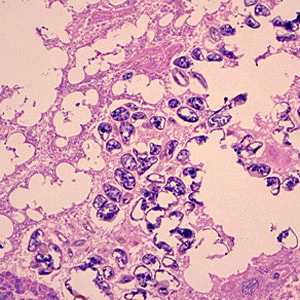
Figure B: Eggs of Diphyllobothrium sp. from the same specimen in Figure A. Image taken at 100x magnification.
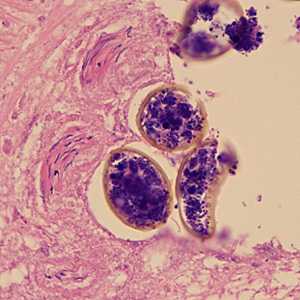
Figure C: Higher magnification (500x) of eggs of Diphyllobothrium sp. from the same specimen in Figures A and B.
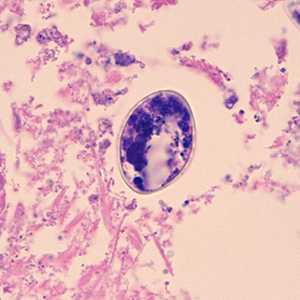
Figure D: Higher magnification (500x) of eggs of Diphyllobothrium sp. from the same specimen in Figures A and B.
Laboratory Diagnosis
Microscopic identification of eggs in the stool is the basis of specific diagnosis. Eggs are usually numerous and can be demonstrated without concentration techniques. Examination of proglottids passed in the stool is also of diagnostic value.
Morphology
More on: Morphologic comparison with other intestinal parasites.
Treatment Information
Praziquantel*, adults, 5-10 mg/kg orally in a single-dose therapy; the dosage for children is the same.
(Note: praziquantel should be taken with liquids during a meal.)
Alternative:
Adults, niclosamide 2 gm orally once; children, 50 mg/kg (max 2 gm) orally once.
(Note: niclosamide must be chewed thoroughly or crushed and swallowed with a small amount of water.)
Praziquantel
Oral praziquantel is available for human use in the United States.
Note on Treatment in Pregnancy
Niclosamide
Niclosamide is NOT available for human use in the United States.
Note on Treatment in Pregnancy
*Not FDA-approved for this indication.
References
- Praziquantel. Cestode (Tapeworm) Infections. In: AHFS Drug Information, McEvoy GK, Ed., American Society of Health-System Pharmacists, Bethesda, MD , 2011.
- Drugs for Parasitic Infections. Treatment Guidelines from the Medical Letter. Vol 8 (Suppl), 2010. The Medical Letter, Inc., New Rochelle, NY.
- Scholz T, Garcia HH, Kuchta R, Wich B. Update on the human broad tapeworm (Genus Diphyllobothrium) including clinical relevance. Clin Microbiol Rev 2009;22:146-60.
- Richards FO Jr. Diphyllobothrium species. In: Long SS, Pickering LK, Prober CG, Eds. Principals and Practices of Pediatric Infectious Diseases Revised Reprint, 3rd Ed, Churchill Livingston/Elsevier, Philadelphia, PA, 2009.
- King CH, Fairley JK. Cestodes. In: Mandell GL, Bennett JE, Dolin R, Eds., Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 7th Ed, Churchill Livingston/Elsevier, Philadelphia, PA, 2009.
- Loukas A, Hotez PJ. Chemotherapy of helminth infections. In: Goodman & Gilman's The Pharmacological Basis of Therapeutics, 11th Ed. McGraw-Hill, NY, 2006.
- Harter A. Milestones of helmintic research at Bayer. Parasitol Res 2002;88:477-80.
- Ohnishi K, Murata M. Praziquantel for the treatment of Diphyllobothrium nihonkaiense infections in humans. Trans R Soc Trop Med Hyg 1994;88:580.
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir