
Cystoisosporiasis
[Cystoisospora belli (syn. Isospora belli)]
Causal Agents
The coccidian parasite, Cystoisospora belli, infects the epithelial cells of the small intestine, and is the least common of the three intestinal coccidia that infect humans.
Life Cycle
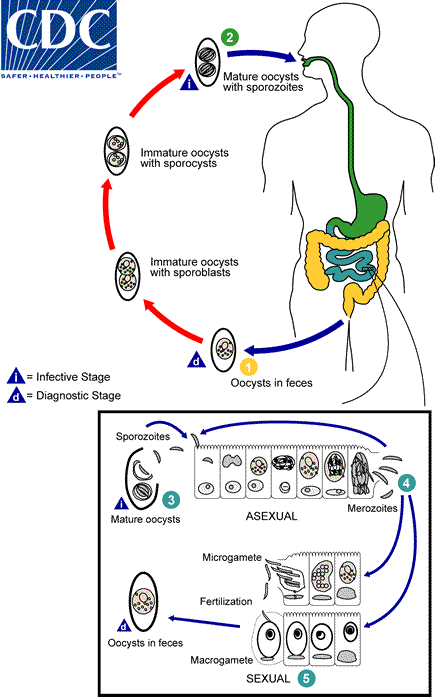
At time of excretion, the immature oocyst contains usually one sporoblast (more rarely two) . In further maturation after excretion, the sporoblast divides in two (the oocyst now contains two sporoblasts); the sporoblasts secrete a cyst wall, thus becoming sporocysts; and the sporocysts divide twice to produce four sporozoites each
. In further maturation after excretion, the sporoblast divides in two (the oocyst now contains two sporoblasts); the sporoblasts secrete a cyst wall, thus becoming sporocysts; and the sporocysts divide twice to produce four sporozoites each . Infection occurs by ingestion of sporocysts-containing oocysts: the sporocysts excyst in the small intestine and release their sporozoites, which invade the epithelial cells and initiate schizogony
. Infection occurs by ingestion of sporocysts-containing oocysts: the sporocysts excyst in the small intestine and release their sporozoites, which invade the epithelial cells and initiate schizogony . Upon rupture of the schizonts, the merozoites are released, invade new epithelial cells, and continue the cycle of asexual multiplication
. Upon rupture of the schizonts, the merozoites are released, invade new epithelial cells, and continue the cycle of asexual multiplication . Trophozoites develop into schizonts which contain multiple merozoites. After a minimum of one week, the sexual stage begins with the development of male and female gametocytes
. Trophozoites develop into schizonts which contain multiple merozoites. After a minimum of one week, the sexual stage begins with the development of male and female gametocytes . Fertilization results in the development of oocysts that are excreted in the stool
. Fertilization results in the development of oocysts that are excreted in the stool .
.
Geographic Distribution
Worldwide, especially in tropical and subtropical areas. Infection occurs in immunodepressed individuals, and outbreaks have been reported in institutionalized groups in the United States.
Clinical Presentation
Infection causes acute, nonbloody diarrhea with crampy abdominal pain, which can last for weeks and result in malabsorption and weight loss. In immunodepressed patients, and in infants and children, the diarrhea can be severe. Eosinophilia may be present (differently from other protozoan infections).
Cystoisospora belli oocysts.
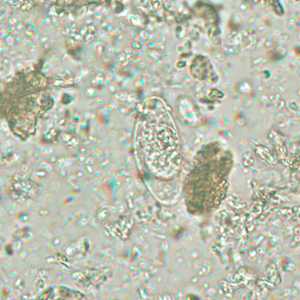
Figure B: Immature oocyst of C. belli stained with safranin, containing a single sporoblast.
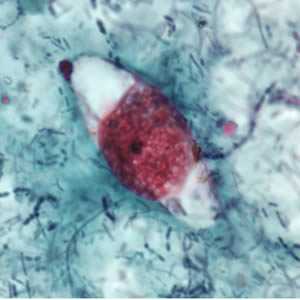
Figure B: Immature oocyst of C. belli stained with safranin, containing a single sporoblast.
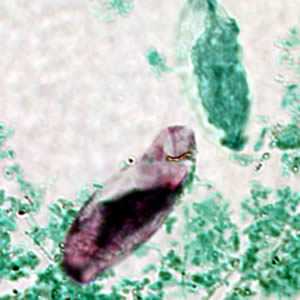
Figure C: Immature oocyst of C. belli stained with acid-fast, showing a single sporoblast.
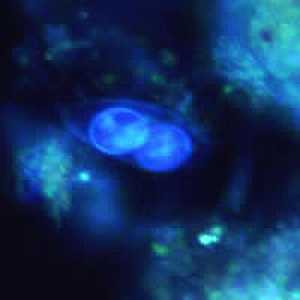
Figure D: Oocyst of C. belli viewed under ultraviolet (UV) microscopy, showing two sporoblasts.
Cystoisospora belli oocysts.
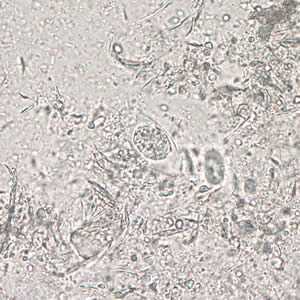
Figure A: Immature oocyst of C. belli in an unstained wet mount showing a single sporoblast.
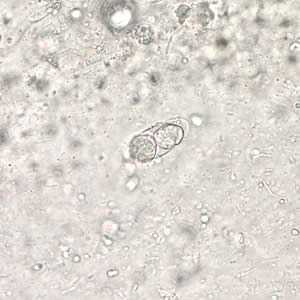
Figure B: Immature oocyst of C. belli in an unstained wet mount showing two sporoblasts.
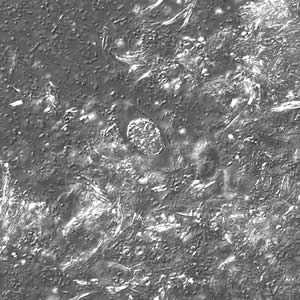
Figure C: Same oocyst as in Figure A but viewed under differential interference contrast (DIC).
Figure D: Same oocyst as in Figure B but viewed under ultraviolet (UV) fluorescent micrscopy.
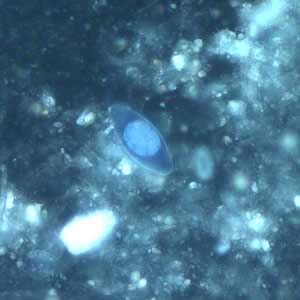
Figure E: Same oocyst as in Figures A and C but viewed under ultraviolet (UV) fluorescent micrscopy.
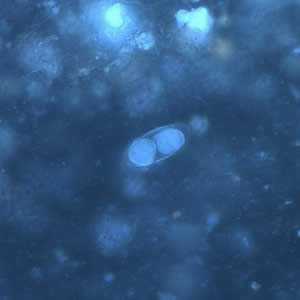
Figure F: Same oocyst as in Figures B and D but viewed under UV microscopy.
Cystoisospora belli oocysts, stained with hematoxylin and eoisin (H&E).
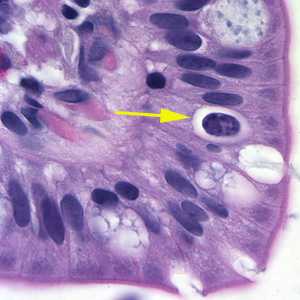
Figure A: Oocyst of C. belli in the epithelial cells of a mammalian host, stained with H&E (yellow arrow).
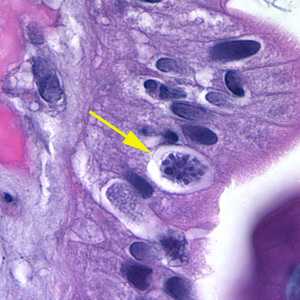
Figure B: Oocyst of C. belli in the epithelial cells of a mammalian host, stained with H&E (yellow arrow).
Laboratory Diagnosis
Microscopic demonstration of the large, typically shaped oocysts, is the basis for diagnosis. Because the oocysts may be passed in small amounts and intermittently, repeated stool examinations and concentration procedures are recommended. If stool examinations are negative, examination of duodenal specimens by biopsy or string test (Enterotest®) may be needed. The oocysts can be visualized on wet mounts by microscopy with bright-field, differential interference contrast (DIC), and epifluorescence. They can also be stained by modified acid-fast stain.
Morphology
More on: Morphological comparisons with other intestinal parasites.
Bench Aids
Treatment Information
Trimethoprim-sulfamethoxazole (TMP-SMX), sold under the trade names Bactrim*, Septra*, and Cotrim*, is the medication of choice for Cystoisospora infection. The typical treatment regimen for adults is TMP 160 mg plus SMX 800 mg (one double-strength tablet), orally, twice a day, for 7 to 10 days.
Expert consultation is recommended if the patient is immunosuppressed, for example, has AIDS. Such patients may need to be treated longer and/or with higher daily doses. This is an example of an alternative regimen of TMP-SMX for adults: one double-strength tablet of TMP-SMX, orally, four times a day, for up to 3 or 4 weeks. Patients with AIDS also may need maintenance therapy (secondary prophylaxis) with TMP-SMX to prevent recurrence of symptomatic infection.
Only limited data are available regarding potential alternatives to TMP-SMX. Patients who are allergic to (or are intolerant of) TMP-SMX usually are treated with pyrimethamine. For adults, the typical daily dose of pyrimethamine is in the range of 50 to 75 mg. This daily dose is given orally, either once a day or divided into 2 separate doses. For example, 50 mg can be given in one dose, or 25 mg can be given twice a day. Pyrimethamine can suppress the bone marrow. To help prevent this, patients treated with pyrimethamine also take leucovorin, orally, in a daily dose in the range of 10 to 25 mg. Folinic acid is another name for leucovorin.
Ciprofloxacin is a second-line alternative. It is less effective than TMP-SMX but might have some activity against Cystoisospora. For adults, the treatment regimen is 500 mg, orally, twice a day, for 7 days.
Trimethoprim–sulfamethoxazole
Trimethoprim–sulfamethoxazole (TMP–SMX) is available for human use in the United States.
Note on Treatment in Pregnancy
Ciprofloxacin
Ciprofloxacin is available for human use in the United States.
Note on Treatment in Pregnancy
* Use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Services.
References
- Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents: Recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR. RR March 24, 2009 / 58(Early Release);1-198.
- Verdier RI, Fitzgerald DW, Johnson WD, Jr, Pape JW. Trimethoprim-sulfamethoxazole compared with ciprofloxacin for treatment and prophylaxis of Isospora belli and Cyclospora cayetanensis infection in HIV-infected patients: a randomized, controlled trial. Ann Intern Med 2000;132:885-8.
- Pape JW, Verdier RI, Johnson WD, Jr. Treatment and prophylaxis of Isospora belli infection in patients with the acquired immunodeficiency syndrome. N Engl J Med 1989;320:1044-7.
- Weiss LM, Perlman DC, Sherman J, et al. Isospora belli infection: treatment with pyrimethamine. Ann Intern Med 1988;109:474-5.
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir
 Key points for laboratory diagnosis of cystoisosporiasis -- Cystoisospora belli
Key points for laboratory diagnosis of cystoisosporiasis -- Cystoisospora belli Key points for laboratory diagnosis of Cyclospora, Cryptosporidium and Cystroisospora belli
Key points for laboratory diagnosis of Cyclospora, Cryptosporidium and Cystroisospora belli