
Cysticercosis
[Taenia solium]
Causal Agents
The larval form (cysticercus) of the pork tapeworm, Taenia solium.
Life Cycle
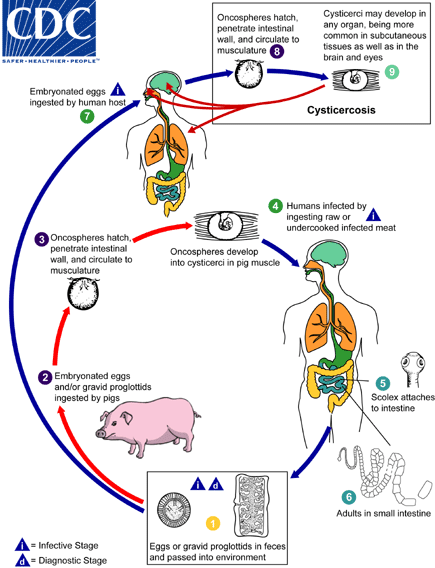
Cysticercosis is an infection of both humans and pigs with the larval stages of the parasitic cestode, Taenia solium. This infection is caused by ingestion of eggs shed in the feces of a human tapeworm carrier . Pigs and humans become infected by ingesting eggs or gravid proglottids
. Pigs and humans become infected by ingesting eggs or gravid proglottids ,
,  . Humans are infected either by ingestion of food contaminated with feces, or by autoinfection. In the latter case, a human infected with adult T. solium can ingest eggs produced by that tapeworm, either through fecal contamination or, possibly, from proglottids carried into the stomach by reverse peristalsis. Once eggs are ingested, oncospheres hatch in the intestine
. Humans are infected either by ingestion of food contaminated with feces, or by autoinfection. In the latter case, a human infected with adult T. solium can ingest eggs produced by that tapeworm, either through fecal contamination or, possibly, from proglottids carried into the stomach by reverse peristalsis. Once eggs are ingested, oncospheres hatch in the intestine ,
,  invade the intestinal wall, and migrate to striated muscles, as well as the brain, liver, and other tissues, where they develop into cysticerci . In humans, cysts can cause serious sequellae if they localize in the brain, resulting in neurocysticercosis. The parasite life cycle is completed, resulting in human tapeworm infection, when humans ingest undercooked pork containing cysticerci
invade the intestinal wall, and migrate to striated muscles, as well as the brain, liver, and other tissues, where they develop into cysticerci . In humans, cysts can cause serious sequellae if they localize in the brain, resulting in neurocysticercosis. The parasite life cycle is completed, resulting in human tapeworm infection, when humans ingest undercooked pork containing cysticerci . Cysts evaginate and attach to the small intestine by their scolex
. Cysts evaginate and attach to the small intestine by their scolex . Adult tapeworms develop, (up to 2 to 7 m in length and produce less than 1000 proglottids, each with approximately 50,000 eggs) and reside in the small intestine for years
. Adult tapeworms develop, (up to 2 to 7 m in length and produce less than 1000 proglottids, each with approximately 50,000 eggs) and reside in the small intestine for years .
.
Geographic Distribution:
Taenia solium is found worldwide. Because pigs are intermediate hosts of the parasite, completion of the life cycle occurs in regions where humans live in close contact with pigs and eat undercooked pork. Taeniasis and cysticercosis are very rare in Muslim countries. It is important to note that human cysticercosis is acquired by ingesting T. solium eggs shed in the feces of a human T. solium tapeworm carrier, and thus can occur in populations that neither eat pork nor share environments with pigs.
Clinical Presentation
The symptoms of cysticercosis are caused by the development of cysticerci in various sites. Of greatest concern is cerebral cysticercosis (or neurocysticercosis), which can cause diverse manifestations including seizures, mental disturbances, focal neurologic deficits, and signs of space-occupying intracerebral lesions. Death can occur suddenly. Extracerebral cysticercosis can cause ocular, cardiac, or spinal lesions with associated symptoms. Asymptomatic subcutaneous nodules and calcified intramuscular nodules can be encountered.
Larval Taenia solium.
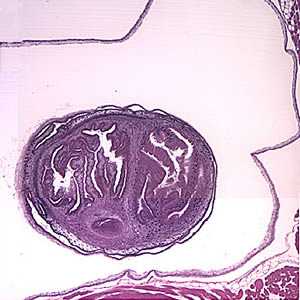
Figure A: Larval Taenia solium cyst in a section of a lesion found in the right frontal lobe of a patient stained with hematoxylin and eosin (H&E), magnification 40×.
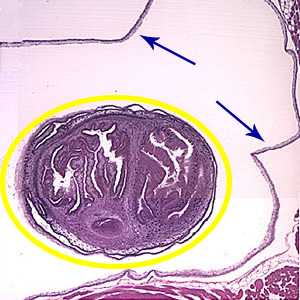
Figure B: An entire cysticercus seen within the bladder walls (blue arrows). A single scolex is visible inside yellow circle) within the cyst.
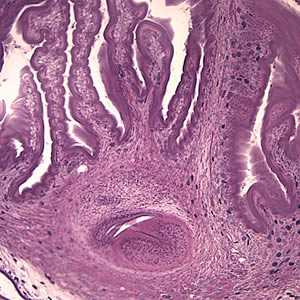
Figure C: Higher magnification (100×) of the cyst in Figures A and B. The parenchymatous portion of the cysticercus can be better observed.
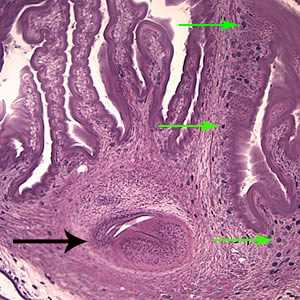
Figure D: The extensive folding of the spiral canal and one sucker of the scolex (black arrow) are apparent. Calcareous corpuscles can be seen in the fibrous tissues (green arrows).
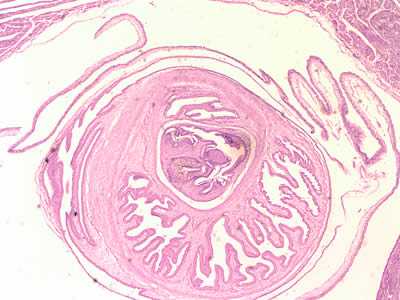
Figure E: Cross-sections of cysticerci stained with H&E, at 40x magnification
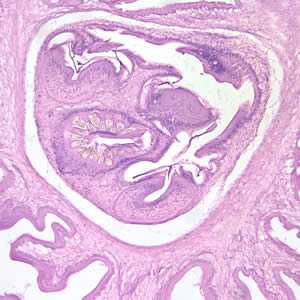
Figure F: Cross-sections of cysticerci stained with H&E, at 100x magnification.
Laboratory Diagnosis
The definitive diagnosis consists of demonstrating the cysticercus in the tissue involved. Demonstration of Taenia solium eggs and proglottids in the feces diagnoses taeniasis and not cysticercosis. Persons who are found to have eggs or proglottids in their feces should be evaluated serologically since autoinfection, resulting in cysticercosis, can occur.
Antibody Detection

Typical antibody reactions in CDC's immunoblot for cysticercosis. Individual sera from patients with either cysticercosis were analyzed using the CDC immunoblot for cysticercosis. Cysticercosis-specific antibodies react with glycoproteins derived from T. solium cysts. The relative molecular masses (Mr) of the seven diagnostic glycoproteins are expressed in kDa and marked with arrows. A positive result is a reaction with any one of the seven cysticercosis-specific proteins.
CDC's immunoblot assay with purified Taenia solium antigens has been acknowledged by the World Health Organization and the Pan American Health Organization as the immunodiagnostic test of choice for confirming a clinical and radiologic presumptive diagnosis of neurocysticercosis. CDC's immunoblot is based on detection of antibodies to one or more of 7 lentil-lectin glycoprotein antigens from the cysticerci of T. solium. In cases where two or more cysts are present, this assay is very sensitive, 100% and 95%, using serum or CSF, respectively, and is essentially 100% specific for either sample. The most important factors contributing to positive immunoblot reactions are the numbers and stage of cysticerci. Cumulative clinical experience has confirmed that in patients with multiple (more than two) lesions, the test has more than 95% sensitivity. Seropositivity in biopsy-confirmed patients with single, enhancing parenchymal cysts was < 50 to 70%%. Seropositivity of patients with multiple but only calcified cysts was 82% in serum and 77% in CSF. In all patients, regardless of their clinical presentation, the immunoblot assay is slightly more sensitive in serum than in CSF specimens; consequently, there is no need to obtain CSF solely for use in the immunoblot assay.
CDC's immunoblot is both more specific and more sensitive than enzyme immunoassays (EIAs). Lack of specificity has been a major problem in most EIAs because of cross-reacting components in crude antigens derived from cysticerci; these components react with antibodies produced in other helminthic infections, especially echinococcosis and filariasis. All positives and any negative strongly suspected of cysticercosis should be confirmed by immunoblot. Currently available antibody detection tests for cysticercosis do not distinguish between active and inactive infections and thus have not been useful in evaluating the outcomes and prognoses of medically treated patients. The CDC immunoblot is commercially available in the United States.
Antigen Detection
Tests that detect circulating cysticercal antigens in serum and CSF have been developed and may prove to be most useful to follow response to therapy in in subarachnoid and ventricular forms of neurocysticercosis. Antigen levels drop quickly in cured NCC patients, so serum antigen monitoring is useful for assessing treatment and determining of clinical cases. Antigen detection testing is not as sensitive as antibody detection and should not be used to diagnose neurocysticercosis. Antigen testing is available at CDC in specific instances and can be ordered after consultation, which can be arranged by contacting CDC (DPDx).
Molecular Detection
PCR tests have been developed to detect T. solium DNA in CSF but these are not widely used for clinical laboratory diagnosis of neurocysticercosis.
Reference
Rodriguez S, Wilkins P, Dorny P. Immunological and molecular diagnosis of cysticercosis. Pathogens and Global Health 2012; 106:286-298.
Treatment Information
The choice of treatment for neurocysticercosis depends on the clinical manifestations and the location, number, size and stage of cysticerci. Anthelminthic chemotherapy for symptomatic neurocysticercosis is almost never a medical emergency. The focus of initial therapy is control of seizures, edema, intracranial hypertension, or hydrocephalus, when one of these conditions is present. Under certain circumstances, a ventricular shunt or other neurosurgical procedure may be indicated. Rarely, neurocysticercosis — especially large and/or subarachnoid (racemose) lesions — may present with imminent threat of intracranial herniation, a neurosurgical emergency.
Anthelminthic therapy, because it kills viable cysts and provokes an inflammatory response, may actually increase symptoms acutely. Co-administration of corticosteroids that cross the blood brain barrier (e.g. dexamethasone) is used to mitigate these effects. Recent placebo-controlled trials confirm that albendazole treatment in appropriately selected neurocysticercosis patients is effective in decreasing the frequency of generalized seizures in long-term follow-up.
Although the heterogeneity of the clinical picture of neurocysticercosis requires individual tailoring of treatment and management, several general principles apply:
- Anthelminthic therapy is generally indicated for symptomatic patients with multiple, live (noncalcified) cysticerci.
- Anthelminthic treatment will not benefit patients with dead worms (calcified cysts).
- Concomitant administration of steroids (e.g. dexamethasone) is often indicated to suppress the inflammatory response induced by destruction of live cysticerci.
- Conventional anticonvulsant therapy is the mainstay of management of neurocysticercosis-associated seizure disorders.
- Intraventricular cysts should usually be treated by surgical removal (endoscopic if possible). Anthelminthics are relatively contraindicated, because the resulting inflammatory response could precipitate obstructive hydrocephalus.
- Although our understanding of subarachnoid neurocysticercosis is evolving, treatment with both anthelminthics and corticosteroids is usually required. Ventricular shunting is often necessary as well.
Even when anthelminthic therapy is successful, continued use of anticonvulsant and other symptomatic medications may still be needed because the pathology may be irreversible. Decisions regarding discontinuation of anticonvulsant regimens must be made on an individual clinical basis, but data suggest that many patients can be eventually weaned from anticonvulsant therapy.
Drugs
Several studies suggest that albendazole (conventional dosage 15 mg/kg/day in 2 divided doses for 15 days) may be superior to praziquantel (50 mg/kg/day for 15 days) for the treatment of neurocysticercosis. In comparative clinical trials, albendazole was equivalent or superior to praziquantel in reducing the number of live cysticerci. A recent placebo-controlled, double-blinded trial demonstrated that albendazole treatment (400 mg twice daily plus 6 mg dexamethasone QD for 10 days) significantly decreased generalized seizures over 30 months of follow-up.
More prolonged treatment courses (e.g. 30 days of albendazole, which may be repeated) may be needed for extraparenchymal or extensive disease. Albendazole is more likely to be effective against extraparenchymal forms of the disease because of better penetration than praziquantel into the CSF. Another possible contributing factor to the greater efficacy of albendazole is that serum and CSF metabolite levels appear to be potentiated by concomitant corticosteroids, whereas praziquantel levels are depressed. Albendazole, unlike praziquantel, has been reported to be effective in giant subarachnoid cysticerci (racemose cysts) and in extraocular muscle cysts. Both drugs appear to have a role in therapy, since cases that have not responded to one of the drugs have been reported to respond to the other.
Albendazole
Oral albendazole is available for human use in the United States.
Note on Treatment in Pregnancy
Note on Treatment During Lactation
Praziquantel
Oral praziquantel is available for human use in the United States.
Note on Treatment in Pregnancy
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir