
Cyclosporiasis
[Cyclospora cayetanensis]
Causal Agents
Cyclospora cayetanensis, a coccidian protozoan. It appears that all human cases are caused by this species.
References:
- Herwaldt BL. Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clin Infect Dis 2000;31:1040-1057.
- Ortega YR, Gilman RH, Sterling CR. A new coccidian parasite (Apicomplexa: Eimeriidae) from humans. J Parasitol 1994;80:625-629.
- Pieniazek NJ, Herwaldt BL. Reevaluating the molecular taxonomy: Is human-associated Cyclospora a mammalian Eimeria species? Emerg Infect Dis 1997;3:381-383.
Life Cycle
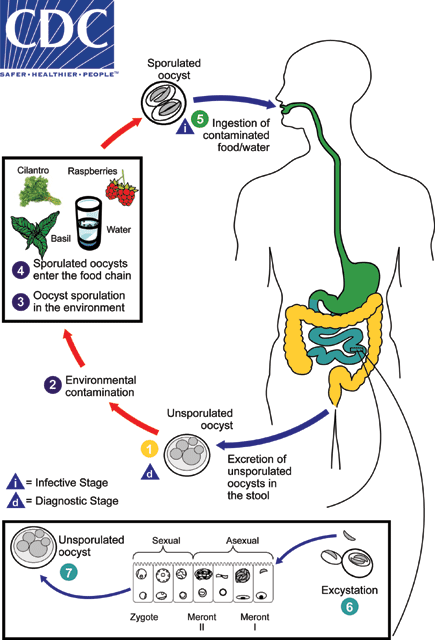
When freshly passed in stools, the oocyst is not infective  (thus, direct fecal-oral transmission cannot occur; this differentiates Cyclospora from another important coccidian parasite, Cryptosporidium). In the environment
(thus, direct fecal-oral transmission cannot occur; this differentiates Cyclospora from another important coccidian parasite, Cryptosporidium). In the environment  , sporulation occurs after days or weeks at temperatures between 22°C to 32°C, resulting in division of the sporont into two sporocysts, each containing two elongate sporozoites
, sporulation occurs after days or weeks at temperatures between 22°C to 32°C, resulting in division of the sporont into two sporocysts, each containing two elongate sporozoites  . Fresh produce and water can serve as vehicles for transmission
. Fresh produce and water can serve as vehicles for transmission  and the sporulated oocysts are ingested (in contaminated food or water)
and the sporulated oocysts are ingested (in contaminated food or water)  . The oocysts excyst in the gastrointestinal tract, freeing the sporozoites which invade the epithelial cells of the small intestine
. The oocysts excyst in the gastrointestinal tract, freeing the sporozoites which invade the epithelial cells of the small intestine  . Inside the cells they undergo asexual multiplication and sexual development to mature into oocysts, which will be shed in stools
. Inside the cells they undergo asexual multiplication and sexual development to mature into oocysts, which will be shed in stools  . The potential mechanisms of contamination of food and water are still under investigation.
. The potential mechanisms of contamination of food and water are still under investigation.
Geographic Distribution
Cyclosporiasis has been reported in many countries, but is most common in tropical and subtropical areas. Since 1990, at least 11 foodborne outbreaks of cyclosporiasis, affecting approximately 3600 persons, have been documented in the United States and Canada.
Clinical Presentation
After an average incubation period of 1 week, symptomatic infections typically manifest as watery diarrhea, which can be severe. Other symptoms include anorexia, weight loss, abdominal pain, nausea and vomiting, myalgias, low-grade fever, and fatigue. Untreated infections typically last for 10-12 weeks and may follow a relapsing course. Infections, especially in disease-endemic settings can be asymptomatic.
Cyclospora cayetanensis oocysts in wet mounts.
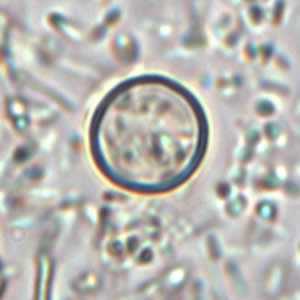
Figure A: Oocyst of C. cayetanensis in an unstained wet mount. Image courtesy of the Oregon State Public Health Laboratory.

Figure B: Oocyst of C. cayetanensis in an unstained wet mount. Image courtesy of the Oregon State Public Health Laboratory.
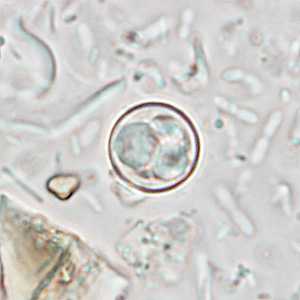
Figure C: Oocyst of C. cayetanensis in an unstained wet mount of stool. Image taken at 1000x magnification.
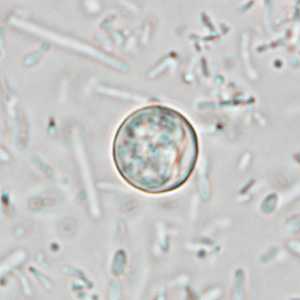
Figure D: Oocyst of C. cayetanensis in an unstained wet mount of stool. Image taken at 1000x magnification.
C. cayetanensis oocysts stained with trichrome.
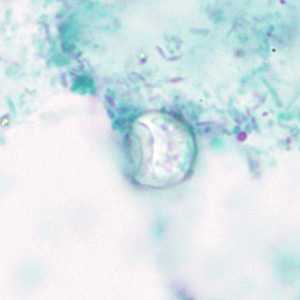
Figure A: Oocyst of C. cayetanensis? stained with trichrome; while the oocyst is visible, the staining characteristics are inadequate for a reliable diagnosis.
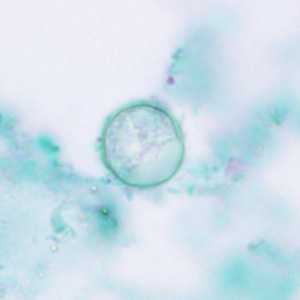
Figure B: Oocysts of C. cayetanensis stained with trichrome; while the oocyst is visible, the staining characteristics are inadequate for a reliable diagnosis.
C. cayetanensis oocysts viewed under ultraviolet (UV) microscopy.

Figure A: Oocyst of C. cayetanensis viewed under UV microscopy.

Figure B: Oocyst of C. cayetanensis viewed under UV microscopy.

Figure C: Oocyst of C. cayetanensis viewed under UV microscopy.

Figure D: Oocyst of C. cayetanensis viewed under UV microscopy.

Figure E: Oocyst of C. cayetanensis viewed under UV microscopy.

Figure F: Oocyst of C. cayetanensis viewed under UV microscopy.
C. cayetanensis oocysts stained with modified acid-fast.
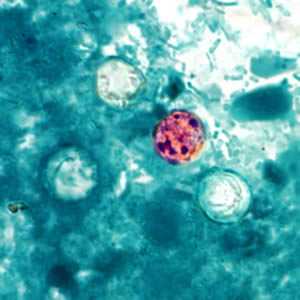
Figure A: Oocysts of C. cayetanensis stained with modified acid-fast stain. Note the variability of staining in the four oocysts.
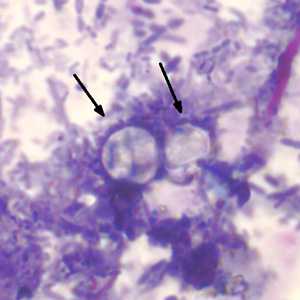
Figure B: Two oocysts of C. cayetanensis stained with modified acid-fast stain. Both oocysts failed to take up the carbol fuschin stain. Image courtesy of the Arizona State Public Health Laboratory.
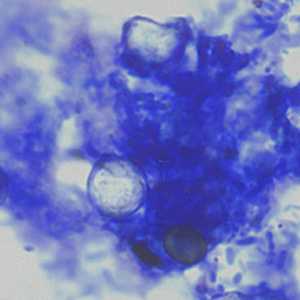
Figure C: Oocysts of C. cayetanensis stained with modified acid-fast stain. Note the wrinkled edge and the lack of stain in the two oocysts. Image courtesy of the Arizona State Public Health Laboratory.
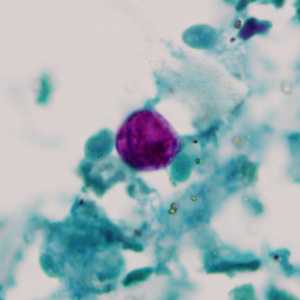
Figure D: Oocyst of C. cayetanensis stained with modified acid-fast stain.
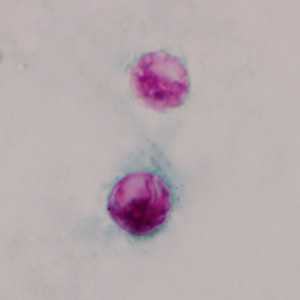
Figure E: Oocysts of C. cayetanensis stained with modified acid-fast stain.
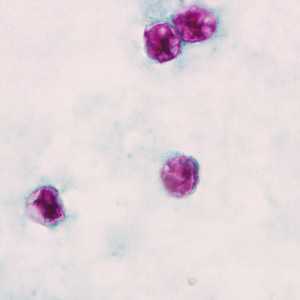
Figure F: Oocysts of C. cayetanensis stained with modified acid-fast stain.
C. cayetanensis oocysts stained with safranin (SAF).
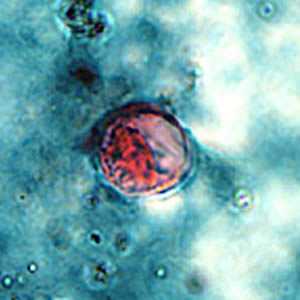
Figure A: Oocyst of C. cayetanensis stained with safranin (SAF).
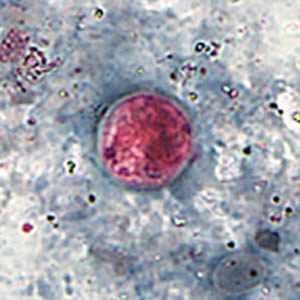
Figure B: Oocyst of C. cayetanensis stained with safranin (SAF).
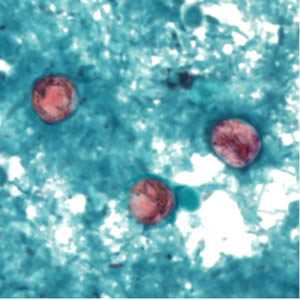
Figure C: Oocyst of C. cayetanensis stained with safranin (SAF).
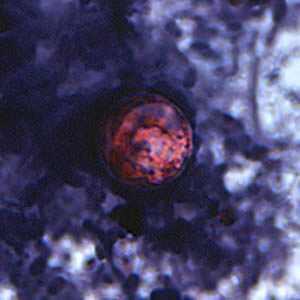
Figure D: Oocyst of C. cayetanensis stained with safranin (SAF).
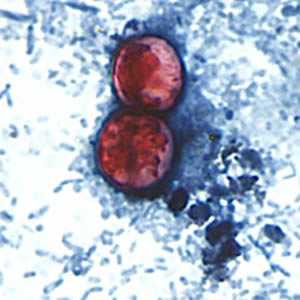
Figure E: A pair of oocysts of C. cayetanensis stained with safranin (SAF).
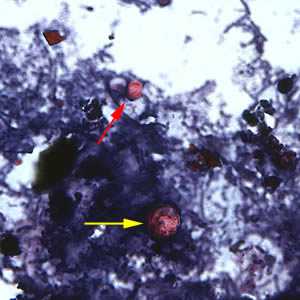
Figure F: Oocyst of C. cayetanensis (yellow arrow) along with an oocyst of Cryptosporidium parvum (red arrow), stained with safranin (SAF). Cryptosporidium spp. also stain with the safranin and modified acid-fast stains.
C. cayetanensis oocysts viewed under differential interference contrast (DIC) microscopy.
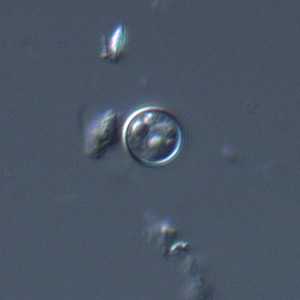
Figure A: Oocyst of C. cayetanensis viewed under differential interference contrast (DIC) microscopy. The refractile globules are easily visible under DIC.

Figure B: Oocyst of C. cayetanensis viewed under differential interference contrast (DIC) microscopy. The refractile globules are easily visible under DIC.
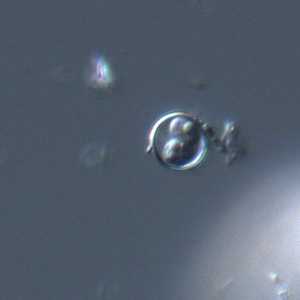
Figure C: Oocyst of C. cayetanensis viewed under DIC microscopy. There are two sporocysts are visible in this image.
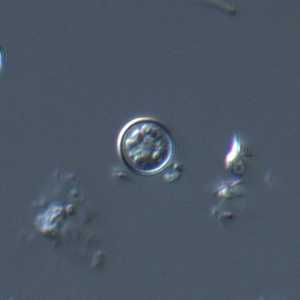
Figure D: Oocyst of C. cayetanensis viewed under DIC microscopy.
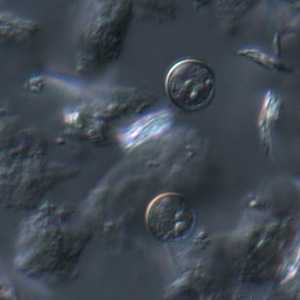
Figure E: A pair of oocysts of C. cayetanensis viewed under DIC microscopy.

Figure F: Rupturing oocyst of C. cayetanensis viewed under DIC microscopy. One sporocyst has has been released from the mature oocyst; the second sporocyst is still contained within the oocyst wall.
Laboratory Diagnosis
Currently, the most practical diagnostic method consists of the identification of oocysts in stool specimens by light microscopy. Although microscopy remains the method of choice in most clinical laboratories, molecular diagnosis using culture-independent diagnostic tests (CIDT), such as the FilmArray Gastrointestinal Panel by BioFire, has become increasingly more common.
Specimen Processing
Specimens should be preserved as soon as possible after collection. Various fixatives can be used depending on what tests will be performed.
- Stool fixed in 10% formalin is recommended for direct microscopy, concentration procedures, and preparation of stained smears; Specimens fixed in sodium acetate-acetic acid formalin can be handled in the same manner as specimens fixed in 10% formalin.
- Stool fixed in formalin-free fixatives, such as polyvinyl alcohol (PVA) or one-vial fixatives (e.g., TotalFix and EcoFix) is suitable forPCR-based detection and UV microscopy.
Unpreserved stool collected in enteric transport media (e.g., Cary-Blair) is commonly used for CIDTs and can be used for confirmatory testing by microscopy and/or PCR if needed. Unpreserved specimens must be refrigerated and sent to the diagnostic laboratory as rapidly as possible.
Cyclospora oocysts can be excreted intermittently and in small numbers. Thus:
- a single negative stool specimen does not rule out the diagnosis; three or more specimens at 2- or 3-day intervals may be required
- concentration procedures should be used to maximize recovery of oocysts. The method most familiar to laboratorians is the formalin-ethyl acetate sedimentation technique (centrifuge for 10 minutes at 500 × g). Other methods can also be used (such as the Sheather’s flotation procedure).
Microscopic Examination
The sediment can be examined microscopically with different techniques:
- wet mounts (by conventional light microscopy, which can be enhanced by UV fluorescence microscopy or differential interference contrast [DIC, Nomarsky])
- stained smears (using modified acid-fast stain or a modified safranin stain)
Molecular diagnosis
Several conventional and real-time PCR protocols have been developed to specifically detect Cyclospora cayetanensis in stool. CDC utilizes a real-time PCR that targets a region of the 18S rRNA gene using a species-specific TaqMan probe. When detecting C. cayetanensis in stool using PCR it is important to choose an appropriate DNA extraction technique that will adequately break open the oocysts; methods that include a vigorous bead beating step generally perform best.
Reference:
- Eberhard ML, Pieniazek NJ, and Arrowood MJ. Laboratory diagnosis of Cyclospora infections. Arch Pathol Lab Med 1997;121:792-797.
- Verveij JJ, Laeijendecker D, Brienen EAT, van Lieshout L, Polderman, AM. Detection of Cyclospora cayetanensis in travellers returning from the tropics. International J Med Microbiol 2003: 293:199-202.
Treatment Information
Trimethoprim-sulfamethoxazole (TMP-SMX), or Bactrim*, Septra*, or Cotrim*, is the treatment of choice. The typical regimen for immunocompetent adults is TMP 160 mg plus SMX 800 mg (one double-strength tablet), orally, twice a day, for 7-10 days. HIV-infected patients may need longer courses of therapy.
No highly effective alternatives have been identified for persons who are allergic to (or are intolerant of) TMP-SMX. Approaches to consider for such persons include observation and symptomatic treatment, use of an antibiotic whose effectiveness against Cyclospora is based on limited data, or desensitization to TMP-SMX. The latter approach should be considered only for selected patients who require treatment, have been evaluated by an allergist, and do not have a life-threatening allergy.
Anecdotal or unpublished data suggest that the following drugs are ineffective: albendazole, trimethoprim (when used as a single agent), azithromycin, nalidixic acid, tinidazole, metronidazole, quinacrine, tetracycline, doxycycline, and diloxanide furoate. Although data from a small study among HIV-infected patients in Haiti suggested that ciprofloxacin might have modest activity against Cyclospora, substantial anecdotal experience among many immunocompetent persons suggests that ciprofloxacin is ineffective.
Trimethoprim–sulfamethoxazole
Trimethoprim–sulfamethoxazole (TMP–SMX) is available for human use in the United States.
Note on Treatment in Pregnancy
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: August 7, 2017
- Page last updated: August 22, 2017
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir
 Key points for laboratory diagnosis of cyclosporiasis — Cyclospora cayetanensis
Key points for laboratory diagnosis of cyclosporiasis — Cyclospora cayetanensis Key points for laboratory diagnosis of Cyclospora, Cryptosporidium, and Cystoisospora belli
Key points for laboratory diagnosis of Cyclospora, Cryptosporidium, and Cystoisospora belli