
Cryptosporidiosis
[Cryptosporidium spp.]
Causal Agents
Many species of Cryptosporidium exist that infect humans and a wide range of animals. Although Cryptosporidium parvum and Cryptosporidium hominis (formerly known as C. parvum anthroponotic genotype or genotype 1) are the most prevalent species causing disease in humans, infections by C. felis, C. meleagridis, C. canis, and C. muris have also been reported.
Life Cycle:
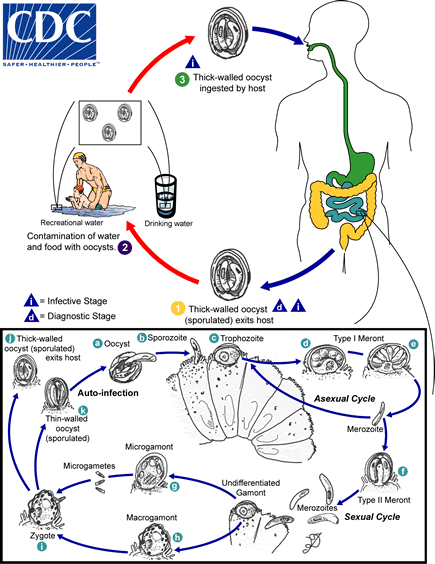
Sporulated oocysts, containing 4 sporozoites, are excreted by the infected host through feces and possibly other routes such as respiratory secretions  . Transmission of Cryptosporidium parvum and C. hominis occurs mainly through contact with contaminated water (e.g., drinking or recreational water). Occasionally food sources, such as chicken salad, may serve as vehicles for transmission. Many outbreaks in the United States have occurred in waterparks, community swimming pools, and day care centers. Zoonotic and anthroponotic transmission of C. parvum and anthroponotic transmission of C. hominis occur through exposure to infected animals or exposure to water contaminated by feces of infected animals
. Transmission of Cryptosporidium parvum and C. hominis occurs mainly through contact with contaminated water (e.g., drinking or recreational water). Occasionally food sources, such as chicken salad, may serve as vehicles for transmission. Many outbreaks in the United States have occurred in waterparks, community swimming pools, and day care centers. Zoonotic and anthroponotic transmission of C. parvum and anthroponotic transmission of C. hominis occur through exposure to infected animals or exposure to water contaminated by feces of infected animals  . Following ingestion (and possibly inhalation) by a suitable host
. Following ingestion (and possibly inhalation) by a suitable host  , excystation
, excystation  occurs. The sporozoites are released and parasitize epithelial cells (
occurs. The sporozoites are released and parasitize epithelial cells ( ,
,  ) of the gastrointestinal tract or other tissues such as the respiratory tract. In these cells, the parasites undergo asexual multiplication (schizogony or merogony) (
) of the gastrointestinal tract or other tissues such as the respiratory tract. In these cells, the parasites undergo asexual multiplication (schizogony or merogony) ( ,
,  ,
,  ) and then sexual multiplication (gametogony) producing microgamonts (male)
) and then sexual multiplication (gametogony) producing microgamonts (male)  and macrogamonts (female)
and macrogamonts (female)  . Upon fertilization of the macrogamonts by the microgametes (
. Upon fertilization of the macrogamonts by the microgametes ( ), oocysts (
), oocysts ( ,
,  ) develop that sporulate in the infected host. Two different types of oocysts are produced, the thick-walled, which is commonly excreted from the host
) develop that sporulate in the infected host. Two different types of oocysts are produced, the thick-walled, which is commonly excreted from the host  , and the thin-walled oocyst
, and the thin-walled oocyst  , which is primarily involved in autoinfection. Oocysts are infective upon excretion, thus permitting direct and immediate fecal-oral transmission.
, which is primarily involved in autoinfection. Oocysts are infective upon excretion, thus permitting direct and immediate fecal-oral transmission.
Note that oocysts of Cyclospora cayetanensis, another important coccidian parasite, are unsporulated at the time of excretion and do not become infective until sporulation is completed. Refer to the life cycle of Cyclospora cayentanensis for further details.
Geographic Distribution:
Worldwide. Outbreaks of cryptosporidiosis have been reported in several countries, the most remarkable being a waterborne outbreak in Milwaukee (Wisconsin) in 1993, that affected more than 400,000 people.
Clinical Presentation
Infection with Cryptosporidium sp. results in a wide range of manifestations, from asymptomatic infections to severe, life-threatening illness; incubation period is an average of 7 days (but can range from 2 to 10 days). Watery diarrhea is the most frequent symptom, and can be accompanied by dehydration, weight loss, abdominal pain, fever, nausea and vomiting. In immunocompetent persons, symptoms are usually short lived (1 to 2 weeks); they can be chronic and more severe in immunocompromised patients, especially those with CD4 counts < 200/µl. While the small intestine is the site most commonly affected, symptomatic Cryptosporidium infections have also been found in other organs including other digestive tract organs, the lungs, and possibly conjunctiva.
Cryptosporidium sp. oocysts in a wet mount.
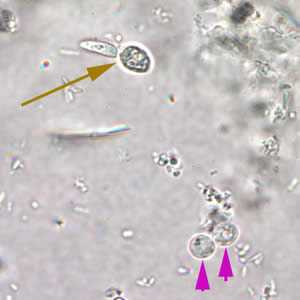
Figure A: Cryptosporidium spp. oocysts (pink arrows) in wet mount. A budding yeast (brown arrow) is in the same field.
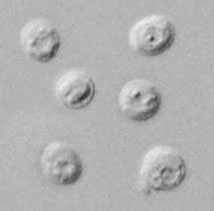
Figure B: Cryptosporidium parvum oocysts in wet mount, under differential interference contrast (DIC) microscopy. The oocysts are rounded and measure 4.2 µm - 5.4 µm in diameter. Sporozoites are visible inside the oocysts, indicating that sporulation has occurred.
Cryptosporidium sp. oocysts stained with trichrome.
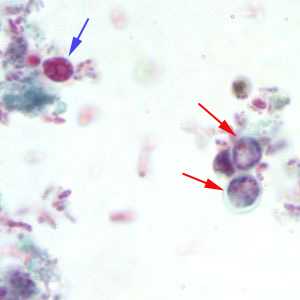
Figure A: Cryptosporidium sp. oocysts stained with trichrome. Oocysts may be detected, but should not be confirmed by this method. Trichrome staining is inadequate for a definite diagnosis because oocysts will appear unstained. Here the Cryptosporidium oocysts are represented by red arrows; the blue arrow represents yeast.
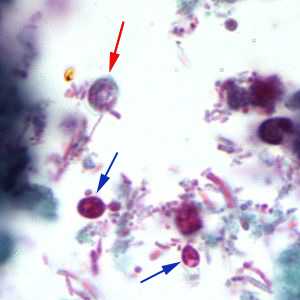
Figure B: Cryptosporidium sp. oocyst stained with trichrome. Oocysts may be detected, but should not be confirmed by this method. Trichrome staining is inadequate for a definite diagnosis because oocysts will appear unstained. Here the Cryptosporidium oocyst is represented by a red arrow; the blue arrows represent yeast.
Cryptosporidium sp. oocysts stained with modified acid-fast.
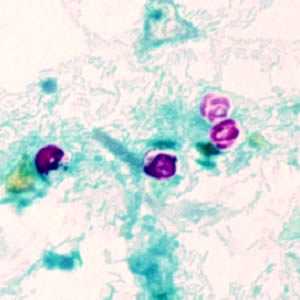
Figure A: Cryptosporidium parvum oocysts stained with modified acid-fast. Against a blue-green background, the oocysts stand out in a bright red stain. Sporozoites are visible inside the two oocysts to the right in this image.

Figure B: Cryptosporidium parvum oocysts stained with modified acid-fast. Against a blue-green background, the oocysts stand out in a bright red stain.
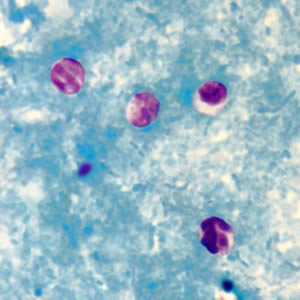
Figure C: Cryptosporidium sp. oocysts stained with modified acid-fast.
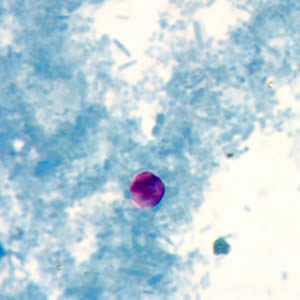
Figure D: Cryptosporidium sp. oocyst stained with modified acid-fast.
Cryptosporidium sp. oocysts unstained on a slide stained with modified acid-fast.
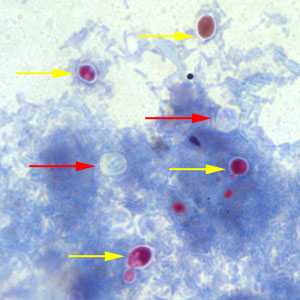
Figure A: Cryptosporidium sp. oocysts (red arrows) that did not take up the modified acid-fast stain. The slide was counterstained with methylene blue. Note that yeast cells did stain red (yellow arrows).
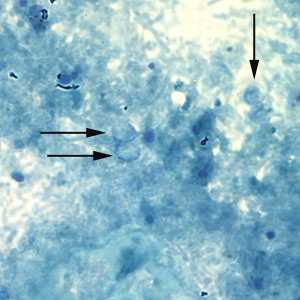
Figure B: Unstained Cryptosporidium sp. oocysts (black arrows) on a slide stained with modified acid-fast. The slide was counterstained with malachite green.
Cryptosporidium sp. oocysts stained with Ziehl-Neelsen modified acid-fast.
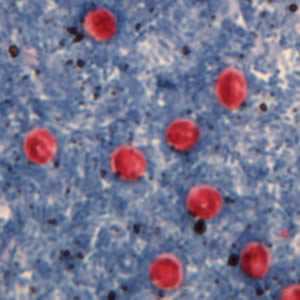
Figure A: Cryptosporidium sp. oocysts stained with Ziehl-Neelson modified acid-fast. Image contributed by the Oregon State Public Health Laboratory.
Cryptosporidium parvum oocysts stained with the fluorescent stain auramine-rhodamine.
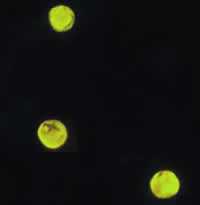
Figure A: Cryptosporidium parvum oocysts stained with the fluorescent stain auramine-rhodamine.
Oocysts of C. parvum and cysts of Giardia duodenalis labeled with immunofluorescent antibodies.
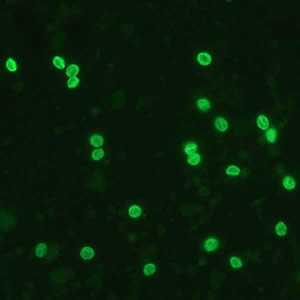
Figure A: Cryptosporidium sp. oocysts labeled with immunofluorescent antibodies. Images contributed by the Kansas Department of Health and Environment.
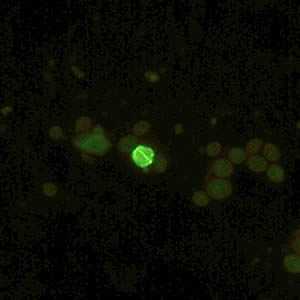
Figure B: Cryptosporidium sp. oocyst labeled with immunofluorescent antibodies. Images contributed by the Kansas Department of Health and Environment.
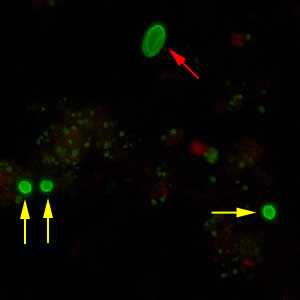
Figure C: Cryptosporidium sp. oocysts (yellow arrows) and cysts of Giardia duodenalis (red arrow) labeled with immunofluorescent antibodies.
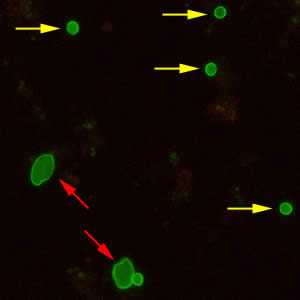
Figure D: Cryptosporidium sp. oocysts (yellow arrows) and cysts of Giardia duodenalis (red arrows) labeled with immunofluorescent antibodies.
Laboratory Diagnosis
Acid-fast staining methods, with or without stool concentration, are most frequently used in clinical laboratories. For greatest sensitivity and specificity, immunofluorescence microscopy is the method of choice (followed closely by enzyme immunoassays). Molecular methods are mainly a research tool.
Safety
Oocysts in stool specimens (fresh or in storage media) remain infective for extended periods. Thus stool specimens should be preserved in 10% buffered formalin or sodium acetate-acetic acid-formalin (SAF) to render oocysts nonviable. (Contact time with formalin necessary to kill oocysts is not clear; we suggest at least 18 to 24 hours). In addition, the usual safety measures for handling potentially infectious material should be adopted.
For more information on safety, visit CDD's Biosafety Guidelines or Occupational Safety and Health Administration (OSHA)![]() .
.
Specimen processing
Stool specimens may be submitted fresh, preserved in 10% buffered formalin (see above, "Safety"), or suspended in a storage medium composed of aqueous potassium dichromate (2.5% w/v, final concentration). The use of mercuric chloride-containing preservatives (e.g., polyvinyl alcohol, PVA) is not recommended due to incompatibilities with some methodologies and the environmental hazards posed by the disposal of mercury-containing compounds. Oocyst numbers can be quite variable, even in liquid stools. Multiple stool samples should be tested before a negative diagnostic interpretation is reported. To maximize recovery of oocysts, stool samples should be concentrated prior to microscopic examination. Formalin-ethyl acetate sedimentation is the recommended stool concentration method for clinical laboratories. Two potential shortcomings of oocyst concentration techniques are:
- Sedimentation methods are generally performed using low speed centrifugation. Given their small size and mass, cryptosporidial oocysts may become trapped in the ether or ethyl acetate plug and fail to sediment properly. Increased centrifugation speed or time (500 x g, 10 minutes) may be warranted when attempting to recover cryptosporidial oocysts.
- Resolution of cryptosporidial infections is accompanied by increasing numbers of non-acid-fast, oocyst "ghosts." Such oocysts may not float or sediment as expected, giving rise to false-negative results.
Enzyme Immunoassays
At least four commercial EIA tests (see Table below) have been introduced for the detection of cryptosporidial antigens in stool samples. These kits are reportedly superior to conventional microscopic examination (especially acid-fast staining methods) and show good correlation with the monoclonal antibody-based immunofluorescence assays. Kit sensitivities and specificities ranged from 66.3% to 100% and 93% to 100%, respectively (see Table).
| Kit Name (Clinical specimens) |
Manufacturer/distributor | Type of test1 | Sensitivity2 | Specificity2 | Comparison test | Reference |
|---|---|---|---|---|---|---|
| ProSpecT/ Cryptosporidium | Alexon, Inc. | EIA-plate | 97 98 96 94 (97) |
98 98 99.5 99 (98) |
acid-fast stain, IIF3 acid-fast stain M-DIF4 M-DIF4, acid-fast, Color Vue |
1 2 3 4 |
| IDEIA Cryptosporidium | Dako Corp. | EIA-plate | 100 (93.1) |
100 (98.7) |
auramine stain, N-DIF5 | 5 |
| MeriFluor™ Cryptosporidium/Giardia | Meridian Diagnostics, Inc. | DFA, IgG | 100 96 (100) |
100 100 (100) |
acid-fast stain acid-fast, ProSpecT, Color Vue |
6 4 |
| Color Vue Cryptosporidium | Seradyn, Inc. | EIA-plate | 93 76 94 (92) |
93 100 100 (100) |
IIF3 M-DIF4 M-DIF4, acid-fast, ProSpecT |
7 3 4 |
| Cryptosporidium Antigen Detection Microwell ELISA | LMD Laboratories | EIA-plate | 66.3 93 |
99.8 99 |
acid-fast, auramine IIF3 |
8 9 |
- 1 EIA = enzyme immunoassay; DFA = direct immunofluorescence assay, IIF = indirect immunofluorescence assay, NA = not available
- 2 Percent specificity or specificity compared to conventional methods, numbers in parentheses indicate values reported by the manufacturer
- 3 IIF = indirect immunofluorescence (MeriFluor Cryptosporidium/Giardia assay)
- 4 M-DIF = direct immunofluorescence (MeriFluor Cryptosporidium/Giardia assay)
- 5 N-DIF = direct immunofluorescence (DetectIF Cryptosporidium, Shield Diagnostics, Ltd.)
References:
- Xia Z, Sonnad S, Turner S, Marasigan M. Evaluation of a microtiter assay for detection of Cryptosporidium antigen in stool. 92nd Annual Meeting of the American Society for Microbiology, New Orleans, LA. 1992. p. 106.
- Dagan R, Fraser D, El-On J, Kassis I, Deckelbaum R, Turner S. Evaluation of an enzyme immunoassay for the detection of Cryptosporidium spp. in stool specimens from infants and young children in field studies. Am J Trop Med Hyg 1995;52:134.
- Aarnaes SL, Blanding J, Speier S, Forthal D, de la Maza LM, Peterson EM. Comparison of the ProSpecT and Color Vue enzyme-linked immunoassays for the detection of Cryptosporidium in stool specimens. Diagn Microbiol Infect Dis 1994;19:221.
- Kehl KSC, Cicirello H, Havens PL. Comparison of four different methods for detection of Cryptosporidium species. J. Clin Microbiol 1995;33:416.
- Siddons CA, Chapman PA, Rush BA. Evaluation of an enzyme immunoassay kit for detecting Cryptosporidium in faeces and environmental samples. J Clin Pathol 1992;45:479.
- Garcia LS, Shum AC, Bruckner DA. Evaluation of a new monoclonal antibody combination reagent for the direct fluorescent detection of Giardia cysts and Cryptosporidium oocysts in human fecal specimens. J Clin Microbiol 1992;30:3255.
- Sloan LM, Rosenblatt JE. Evaluation of an immunoassay for the detection of Cryptosporidium stool specimens. 91st Annual Meeting of the American Society for Microbiology, Dallas, TX. 1991. p. 22.
- Newman RD, Jaeger KL, Wuhib T, Lima AA, Guerrant RL, Sears CL. Evaluation of an antigen capture enzyme-linked immunosorbent assay for detection of Cryptosporidium oocysts. J Clin Microbiol 1993;31:2080.
- Rosenblatt JE, Sloan LM. Evaluation of an enzyme-linked immunosorbent assay for detection of Cryptosporidium spp. in stool specimens. J Clin Microbiol 1993;31:1468.
Molecular Methods
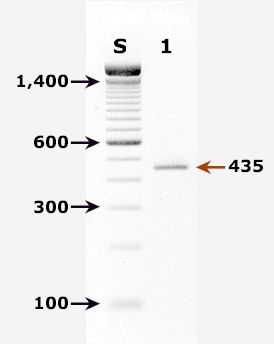
Agarose gel (2%) analysis of a PCR diagnostic test for detection of Cryptosporidium parvum DNA. PCR was performed using primers CPBDIAGF and CPBDIAGR.
Agarose gel (2%) analysis of a PCR diagnostic test for detection of Cryptosporidium parvum DNA. PCR was performed using primers CPBDIAGF and CPBDIAGR.1
- Lane S: Molecular base pair standard (100-bp ladder). Black arrows show the size of standard bands.
- Lane 1: C. parvum positive fecal specimen. The red arrow shows the diagnostic band of 435 bp for zoonotic Cryptosporidium parvum.
Real-Time PCR
A TaqMan-based real-time PCR assay for detection and identification of Cryptosporidium parvum (bovine genotype) and Cryptosporidium hominis (human genotype) has been developed and validated at CDC.2 The assay combines the detection of two genomic targets: the 18S rRNA gene to achieve a sensitive detection of Cryptosporidium spp. and a gene with unknown function to provide species differentiation. Each DNA sample is run in two parallel reactions. The first consists of the highly sensitive detection of the Cryptosporidium 18S rRNA gene and the species-specific detection of C. parvum in a duplex format. The other reaction detects C. hominis on the species level.
More on: TaqMan real-time PCR
References
- Johnson DW, Pieniazek NJ, Griffin DW, Misener L, Rose JB. Development of a PCR protocol for sensitive detection of Cryptosporidium oocysts in water samples. Appl Environ Microbiol 1995;61:3849-55.
Antibody detection: There are currently no commercially available serologic assays for the detection of Cryptosporidium-specific antibodies. However, immunoblots for detecting the 17 and 27 kDa sporozoite antigens associated with recent infection may be useful for epidemiologic investigations.
Treatment Information
Rapid loss of fluids because of diarrhea can be managed by fluid and electrolyte replacement. Infection in healthy, immunocompetent persons is self-limited. Nitazoxanide has been approved for treatment of diarrhea caused by Cryptosporidium in immunocompetent patients. Immunocompromised persons and those in poor health are at highest risk for severe illness. The effectiveness of nitazoxanide in immunosuppressed persons is unclear. For persons with AIDS, anti-retroviral therapy, which improves immune status, will also reduce oocyst excretion and decrease diarrhea associated with cryptosporidiosis. For additional information, see the recommendations in the Guidelines for preventing opportunistic infections among HIV-infected persons -- 2002.
References
- Kaplan JE, Masur H, Holmes KK. Guidelines for preventing opportunistic infections among HIV-infected persons. MMWR June 14, 2002; 51(RR08):1-46.
- Morgan-Ryan UM, Fall A, Ward LA, Hijjawi N, Sulaiman I, Fayer R, et al. Cryptosporidium hominis n. sp. (Apicomplexa: Cryptosporidiidae) from Homo sapiens. J Eukaryot Microbiol 2002;49:433-440.
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir
 Laboratory Diagnosis of Cryptosporidiosis -- Cryptosporidium spp.
Laboratory Diagnosis of Cryptosporidiosis -- Cryptosporidium spp.