
Clonorchiasis
[Clonorchis sinensis]
Causal Agents
The trematode Clonorchis sinensis (Chinese or oriental liver fluke).
Life Cycle
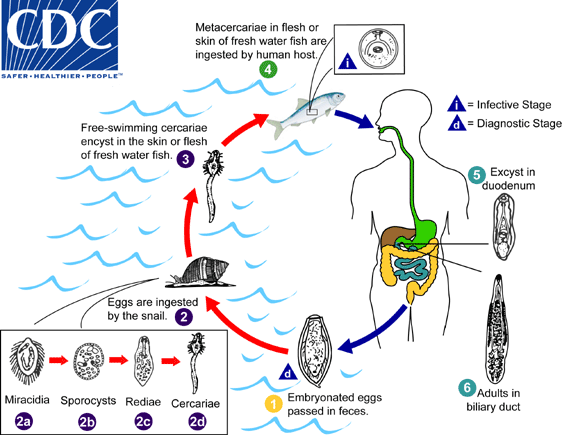
Embryonated eggs are discharged in the biliary ducts and in the stool  . Eggs are ingested by a suitable snail intermediate host
. Eggs are ingested by a suitable snail intermediate host  . Each egg releases a miracidia
. Each egg releases a miracidia  , which go through several developmental stages (sporocysts
, which go through several developmental stages (sporocysts  , rediae
, rediae  , and cercariae
, and cercariae 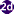 . The cercariae are released from the snail and after a short period of free-swimming time in water, they come in contact and penetrate the flesh of freshwater fish, where they encyst as metacercariae
. The cercariae are released from the snail and after a short period of free-swimming time in water, they come in contact and penetrate the flesh of freshwater fish, where they encyst as metacercariae  Infection of humans occurs by ingestion of undercooked, salted, pickled, or smoked freshwater fish
Infection of humans occurs by ingestion of undercooked, salted, pickled, or smoked freshwater fish  . After ingestion, the metacercariae excyst in the duodenum
. After ingestion, the metacercariae excyst in the duodenum  and ascend the biliary tract through the ampulla of Vater
and ascend the biliary tract through the ampulla of Vater  Maturation takes approximately 1 month. The adult flukes (measuring 10 to 25 mm by 3 to 5 mm) reside in small and medium sized biliary ducts. In addition to humans, carnivorous animals can serve as reservoir hosts.
Maturation takes approximately 1 month. The adult flukes (measuring 10 to 25 mm by 3 to 5 mm) reside in small and medium sized biliary ducts. In addition to humans, carnivorous animals can serve as reservoir hosts.
Geographic Distribution
Endemic areas are in Asia including Korea, China, Taiwan, and Vietnam. Clonorchiasis has been reported in non endemic areas (including the United States). In such cases, the infection is found in Asian immigrants, or following ingestion of imported, undercooked or pickled freshwater fish containing metacercariae.
Clinical Presentation
Most pathologic manifestations result from inflammation and intermittent obstruction of the biliary ducts. In the acute phase, abdominal pain, nausea, diarrhea, and eosinophilia can occur. In long-standing infections, cholangitis, cholelithiasis, pancreatitis, and cholangiocarcinoma can develop, which may be fatal.
Clonorchis sinensis eggs.
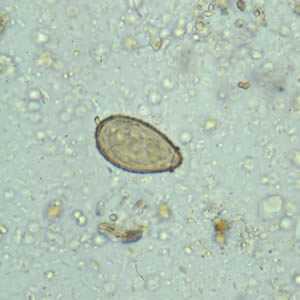
Figure A: C. sinensis egg: the small knob at the abopercular end is visible in this image.
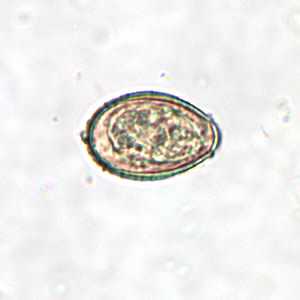
Figure B: C. sinensis egg. Note the operculum resting on "shoulders;" image taken at 400× magnification.
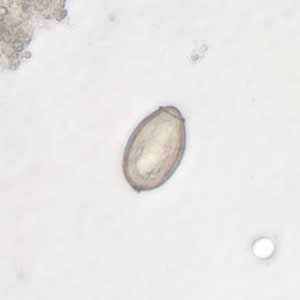
Figure C: C. sinensis egg; images taken at 400× magnification.

Figure D: C. sinensis egg; images taken at 400× magnification.
C. sinensis adults.
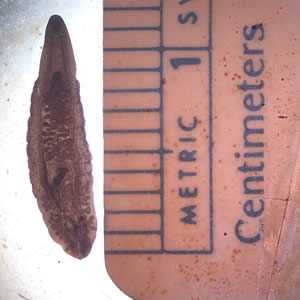
Figure A: Adult of C. sinensis.
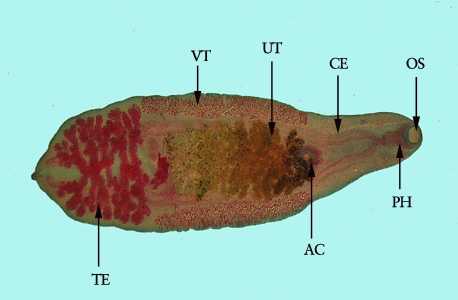
Figure B: Adult of C. sinensis stained with carmine. Clearly visible in this image are the oral sucker (OS), pharynx (PH), ceca (CE), acetabulum, or ventral sucker (AC), uterus (UT), vitellaria (VT) and testes (TE).
Snail intermediate hosts of C. sinensis.
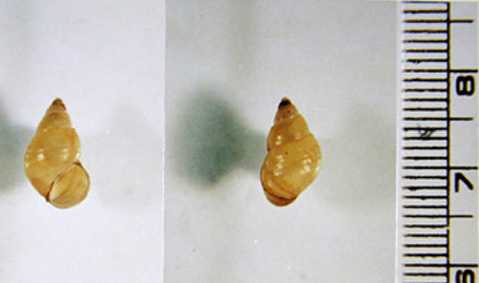
Figure A: Shells of Parafossarulus manchouricus, the most common snail host of C. sinensis in endemic areas in southeast Asia. Image courtesy of the Web Atlas of Medical Parasitology and the Korean Society for Parasitology.

Figure B: Bithynia sp., another common intermediate host of C. sinensis. Image courtesy of Michal Maňas.
Laboratory Diagnosis
Microscopic demonstration of eggs in the stool or in duodenal aspirate is the most practical diagnostic method. The adult fluke can also be recovered at surgery.
Serologic testing is currently not available for clonorchiasis in the United States.
More on: Morphologic comparison with other intestinal parasites.
Treatment Information
Praziquantel, adults, 75mg/kg/day orally, three doses per day for 2 days; the pediatric dosage is the same. Praziquantel should be taken with liquids during meals.
Alternative:
Albendazole* is an alternative drug; the dosage for adults is 10mg/kg/day for 7 days. The pediatric dosage is the same. Albendazole should be taken with food; a fatty meal increases the bioavailablility.
Praziquantel
Oral praziquantel is available for human use in the United States.
Note on Treatment in Pregnancy
Albendazole
Oral albendazole is available for human use in the United States.
Note on Treatment in Pregnancy
Note on Treatment During Lactation
*Not FDA-approved for this indication
References
- Keiser J, Utzinger J. The drugs we have and the drugs we need against major helminth infections. Adv Parasitol 2010;73:197-230.
- Keiser J, Utzinger J. Food-borne tremadodiases. Clin Microbiol Rev 2009;22:466-83.
- Keiser J, Utzinger J. Food-borne trematodiasis: current chemotherapy and advances with artemisinins and synthetic trioxolanes. Trends Parasitol 2007;23:605-12.
- Liu YH, Wang XG, Gao P, Ming-xin Q. Experimental and clinical trial of albendazole in the treatment of clonorchiasis (Clonorchis) sinensis. Chin Med J (Engl) 1991;104:27-31.
- Yangco BG, De Lerma C, Lyman GH, Price DL. Clinical study evaluating efficacy of praziquantel in clonorchiasis. Antimicrob Agents Chemother 1987;31:135-8.
- Jong EC, Wasserheit JN, Johnson RJ, Carberry WL, Agosti J, Dunning S, Clark H. Praziquantel for the treatment of Clonorchis/Opisthorchis infections: report of a double-blind, placebo-controlled trial. J Infect Dis 1985;152:637-40.
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir