
Blastocystis hominis
[Blastocystis hominis]
Causal Agents
The taxonomic classification of Blastocystis hominis is mired in controversy. It has been previously considered as yeasts, fungi, or ameboid, flagellated, or sporozoan protozoa. Recently, however, based on molecular studies, especially dealing with the sequence information on the complete SSUrRNA gene, B. hominis has been placed within an informal group, the stramenopiles (Silberman et al. 1996). Stramenopiles are defined, based on molecular phylogenies, as a heterogeneous evolutionary assemblage of unicellular and multicellular protists including brown algae, diatoms, chrysophytes, water molds, slime nets, etc. (Patterson, 1994). Cavalier-Smith (1998) considers stramenopiles to be identical to his infrakingdom Heterokonta under the kingdom Chromista. Therefore, according to Cavalier-Smith, B. hominis is a heterokontid chromista.
References:
- Silberman JD, Sogin ML, Leipe DD, Clark CG. Human parasite finds taxonomic home. Nature 1996;380:398.
- Patterson DJ. Protozoa, evolution and systematics. In: Housmann K, Hulsmann N, editors. Progress in Protozoology. Stuttgart: Fischer; 1994. p. 1-14.
- Cavalier-Smith T. A revised six-kingdom system of life. Biol Rev Camb Philos Soc 1998;73:203-266.
Life Cycle
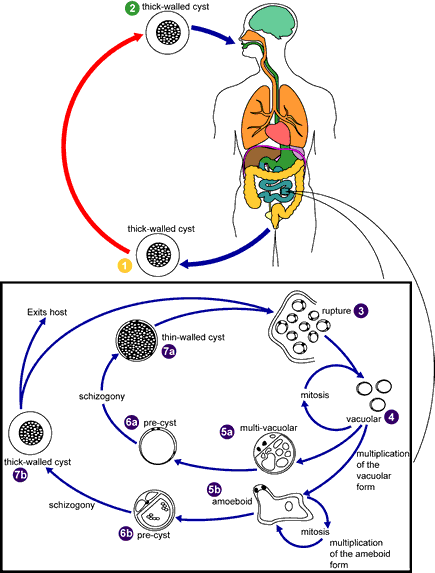
Blastocystis hominis stages were reproduced from Singh M, Suresh K, Ho LC, Ng GC, Yap EH. Elucidation of the life cycle of the intestinal protozoan Blastocystis hominis. Parasitol Res 1995;81:449. Copyright held by Springer-Verlag and reproduced here by permission of Springer-Verlag and M. Singh.
Knowledge of the life cycle and transmission is still under investigation, therefore this is a proposed life cycle for B. hominis. The classic form found in human stools is the cyst, which varies tremendously in size from 6 to 40 µm  . The thick-walled cyst present in the stools
. The thick-walled cyst present in the stools  is believed to be responsible for external transmission, possibly by the fecal-oral route through ingestion of contaminated water or food
is believed to be responsible for external transmission, possibly by the fecal-oral route through ingestion of contaminated water or food  . The cysts infect epithelial cells of the digestive tract and multiply asexually (
. The cysts infect epithelial cells of the digestive tract and multiply asexually ( ,
,  ). Vacuolar forms of the parasite give origin to multi vacuolar
). Vacuolar forms of the parasite give origin to multi vacuolar  and ameboid forms
and ameboid forms  . The multi-vacuolar develops into a pre-cyst
. The multi-vacuolar develops into a pre-cyst  that gives origin to a thin-walled cyst
that gives origin to a thin-walled cyst  , thought to be responsible for autoinfection. The ameboid form gives origin to a pre-cyst
, thought to be responsible for autoinfection. The ameboid form gives origin to a pre-cyst  , which develops into thick-walled cyst by schizogony
, which develops into thick-walled cyst by schizogony  . The thick-walled cyst is excreted in feces
. The thick-walled cyst is excreted in feces  .
.
Geographic Distribution
Worldwide.
Clinical Presentation
Whether Blastocystis hominis can cause symptomatic infection in humans is a point of active debate. This is because of the common occurrence of the organism in both asymptomatic and symptomatic persons. Those who believe symptoms could be related to infection with this parasite have described a spectrum of illness including watery diarrhea, abdominal pain, perianal pruritus, and excessive flatulence.
Blastocystis hominis cyst-like forms in wet mounts.
Blastocystis hominis appear as spherical to oval cyst-like structures. They vary widely in size (5 to 30 µm; usual range 8 to 10 µm), and typically consist of a central body, or "vacuole," surrounded by a thin rim of cytoplasm containing up to six nuclei. The vacuoles stain variably from red to blue in trichrome stain, which is preferred to wet mounts as the organisms can be overlooked as debris.
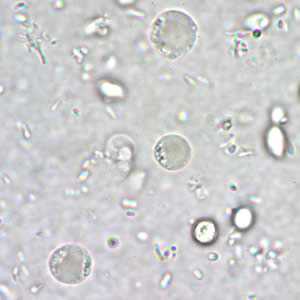
Figure A: B. hominis cyst-like forms in a wet mount, unstained.
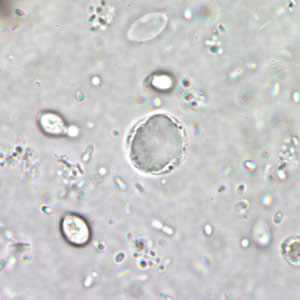
Figure B: B. hominis cyst-like form in a wet mount, unstained.
B. hominis cyst-like forms in wet mounts under differential interference contrast (DIC) microscopy.
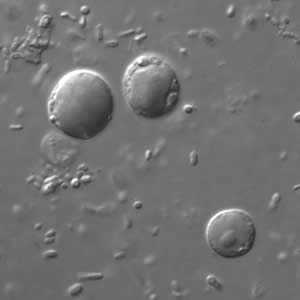
Figure A: B. hominis cyst-like forms in wet mounts under differential interference contrast (DIC) microscopy.
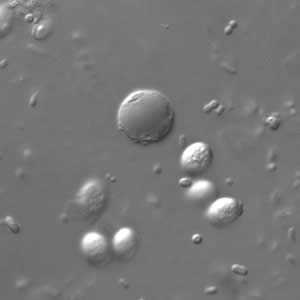
Figure B: B. hominis cyst-like forms in wet mounts under differential interference contrast (DIC) microscopy.
B. hominis cyst-like forms in wet mounts stained with iodine.
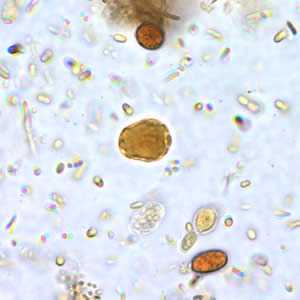
Figure A: B. hominis cyst-like forms in wet mounts stained in iodine.
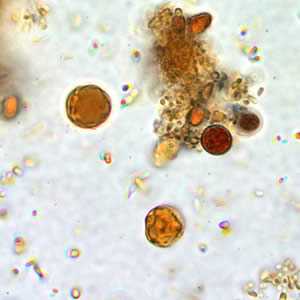
Figure B: B. hominis cyst-like forms in wet mounts stained in iodine.
B. hominis cyst-like forms stained with trichrome
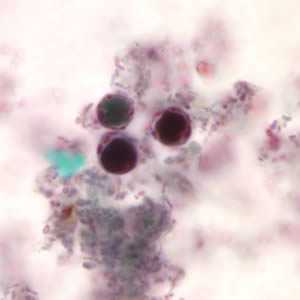
Figure A: B. hominis cyst-like forms stained with trichrome. The nuclei in the peripheral cytoplasmic rim are visible, staining purple.
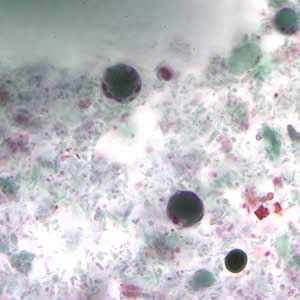
Figure B: B. hominis cyst-like forms stained with trichrome. The nuclei in the peripheral cytoplasmic rim are visible, staining purple.
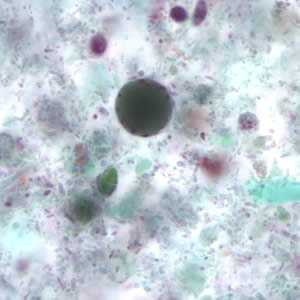
Figure C: Blastocystis hominis cyst-like forms stained with trichrome.
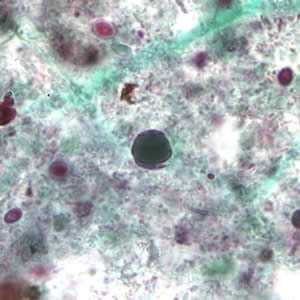
Figure D: Blastocystis hominis cyst-like forms stained with trichrome.
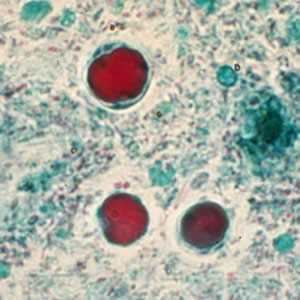
Figure E: B. hominis cyst-like forms stained with trichrome.
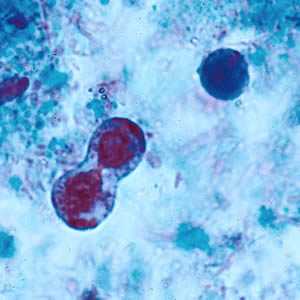
Figure F: B. hominis cyst-like forms undergoing binary fission; stained with trichrome.
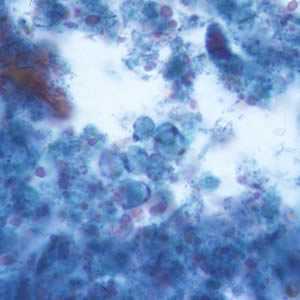
Figure G: B. hominis cyst-like forms stained with trichrome.
Laboratory Diagnosis
Diagnosis is based on finding the cyst-like stage in feces. Permanently stained smears are preferred over wet mount preparations because fecal debris may be mistaken for the organisms in the latter. Do not wash specimens in water (e.g., during concentration procedures) as this will lyse the organisms, resulting in false negatives.
Morphology
More on: Morphologic comparison with other intestinal parasites
Treatment Information
The clinical significance of Blastocystis spp. is controversial.
Treatment with metronidazole* at various doses has been reported, for example (adults):
- 250 mg to 750 mg metronidazole* orally 3 times daily for 10 days
- 1500 mg metronidazole* orally once daily for 10 days
Note: Lack of response to metronidazole has been noted in some areas (Yakoob et al., Br J Biomed Sci 2004;61:75).
Treatment with trimethoprim (TMP)*/sulfamethoxazole (SMX)* at various doses has been reported, for example (adults):
- 6 mg/kg TMP*, 30 mg/kg SMX* once daily for 7 days
- 320mg TMP*, 1600 mg SMX* once daily for 7 days
- 160 mg TMP*, 800 mg SMX* twice daily for 7 days
Treatment with nitazoxanide* has been shown to be effective in clearing organisms and improving symptoms at the following doses:
- Adults, 500 mg nitazoxanide* orally twice daily for 3 days.
- Children, 200 mg nitazoxanide* orally twice daily for 3 days in patients aged 4–11 years, and 100 mg nitazoxanide* orally twice daily for 3 days in patients aged 1–3 years.
Tinidazole*, paromomycin*, iodoquinol*, and ketoconazole* have also been used for clearing Blastocystis, as presented in case reports or small series (see references).
*Not FDA-approved for this indication.
Metronidazole
Metronidazole is available for human use in the United States.
Note on Treatment in Pregnancy
Trimethoprim–sulfamethoxazole
Trimethoprim–sulfamethoxazole (TMP–SMX) is available for human use in the United States.
Note on Treatment in Pregnancy
DPDx is an education resource designed for health professionals and laboratory scientists. For an overview including prevention and control visit www.cdc.gov/parasites/.
- Page last reviewed: May 3, 2016
- Page last updated: May 3, 2016
- Content source:
- Global Health – Division of Parasitic Diseases and Malaria
- Notice: Linking to a non-federal site does not constitute an endorsement by HHS, CDC or any of its employees of the sponsors or the information and products presented on the site.
- Maintained By:


 ShareCompartir
ShareCompartir
 Laboratory diagnosis of Blastocystis hominis infection -- Blastocystis hominis
Laboratory diagnosis of Blastocystis hominis infection -- Blastocystis hominis