Mercury
Mercury Overview
Summary
 Mercury occurs naturally in the environment and exists in several forms.
Mercury occurs naturally in the environment and exists in several forms.
These forms can be organized under three headings: metallic mercury (also known as elemental mercury), inorganic mercury, and organic mercury. Metallic mercury is used in a variety of household products and industrial items, including thermostats, fluorescent light bulbs, barometers, glass thermometers, and some blood pressure devices. Additionally, some religions have practices that may include the use of metallic mercury. The most common organic mercury compound that microorganisms and natural processes generate from other forms is methylmercury. Methylmercury is of particular concern because it can build up in certain edible freshwater and saltwater fish and marine mammals to levels that are many times greater than levels in the surrounding water.
Because mercury occurs naturally in the environment, everyone is exposed to very low levels of mercury in air, water, and food. Some people may be exposed to higher levels of mercury in this form if they have a diet high in fish, shellfish, or marine mammals. Workers are mostly exposed from breathing air that contains mercury vapors, but may also be exposed to other inorganic mercury compounds in the workplace. Children are at risk of being exposed to metallic mercury that is not safely contained, to mercury that may be brought home on work clothes or tools, or to methylmercurycontaminated foods.
The nervous system is very sensitive to mercury. Permanent damage to the brain has been shown to occur from exposure to sufficiently high levels of metallic mercury. The kidneys are also sensitive to the effects of mercury, because mercury accumulates in the kidneys and causes higher exposures to these tissues, and thus more damage.
Be careful when you handle and dispose of all items in the home that contain metallic mercury. If larger amounts of metallic mercury are found (for example, a jar of liquid mercury), it should be contained in an airtight container, and you should call your local health department for instructions on how to safely dispose of it.
There are reliable and accurate ways to measure mercury levels in the body. These tests all involve taking blood, urine, or hair samples, and must be performed in a doctor’s office or in a health clinic. To help prevent harm from exposure, the EPA, FDA, and OSHA have established exposure limits. Top of Page
Mercury and the Environment
 Mercury is a naturally occurring metal found throughout the environment.
Mercury is a naturally occurring metal found throughout the environment.
Mercury enters the environment as the result of the normal breakdown of minerals in rocks and soil from exposure to wind and water, and from volcanic activity. Human activities (e.g., mining, burning of fossil fuels) have resulted in additional release of mercury to the environment. Estimates of the total annual mercury releases that result from human activities range from one-third to two-thirds of the total mercury releases. The levels of mercury in the atmosphere (i.e., the air you breathe in the general environment) are very, very low and do not pose a health risk; however, the steady release of mercury has resulted in current levels that are three to six times higher than the estimated levels in the preindustrial era atmosphere.
Approximately 80% of the mercury released from human activities is elemental mercury released to the air, primarily from fossil fuel combustion, mining, and smelting, and from solid waste incineration. About 15% of the total is released to the soil from fertilizers, fungicides, and municipal solid waste (for example, from waste that contains discarded batteries, electrical switches, or thermometers). An additional 5% is released from industrial wastewater to water in the environment. With the exception of mercury ore deposits, the amount of mercury that naturally exists in any one place is usually very low. In contrast, the amount of mercury that may be found in soil at a particular hazardous waste site because of human activity can be high (over 200,000 times natural levels). The mercury in air, water, and soil at hazardous waste sites may come from both natural sources and human activity.
 Most of the mercury found in the environment is in the form of metallic mercury and inorganic mercury compounds. Metallic and inorganic mercury enters the air from mining deposits of ores that contain mercury, from the emissions of coal-fired power plants, from burning municipal and medical waste, from the production of cement, and from uncontrolled releases in factories that use mercury. Metallic mercury is a liquid at room temperature, but some of the metal will evaporate into the air and 7 can be carried long distances. In air, the mercury vapor can be changed into other forms of mercury, and can be further transported to water or soil in rain or snow. Inorganic mercury may also enter water or soil from the weathering of rocks that contain mercury, from factories or water treatment facilities that release water contaminated with mercury, and from incineration of municipal garbage that contains mercury (for example, in thermometers, electrical switches, or batteries that have been thrown away). Inorganic or organic compounds of mercury may be released to the water or soil if mercury-containing fungicides are used.
Most of the mercury found in the environment is in the form of metallic mercury and inorganic mercury compounds. Metallic and inorganic mercury enters the air from mining deposits of ores that contain mercury, from the emissions of coal-fired power plants, from burning municipal and medical waste, from the production of cement, and from uncontrolled releases in factories that use mercury. Metallic mercury is a liquid at room temperature, but some of the metal will evaporate into the air and 7 can be carried long distances. In air, the mercury vapor can be changed into other forms of mercury, and can be further transported to water or soil in rain or snow. Inorganic mercury may also enter water or soil from the weathering of rocks that contain mercury, from factories or water treatment facilities that release water contaminated with mercury, and from incineration of municipal garbage that contains mercury (for example, in thermometers, electrical switches, or batteries that have been thrown away). Inorganic or organic compounds of mercury may be released to the water or soil if mercury-containing fungicides are used.
 Microorganisms (bacteria, phytoplankton in the ocean, and fungi) convert inorganic mercury to methylmercury. Methylmercury released from microorganisms can enter the water or soil and remain there for a long time, particularly if the methylmercury becomes attached to small particles in the soil or water. Mercury usually stays on the surface of sediments or soil and does not move through the soil to underground water. If mercury enters the water in any form, it is likely to settle to the bottom where it can remain for a long time.
Microorganisms (bacteria, phytoplankton in the ocean, and fungi) convert inorganic mercury to methylmercury. Methylmercury released from microorganisms can enter the water or soil and remain there for a long time, particularly if the methylmercury becomes attached to small particles in the soil or water. Mercury usually stays on the surface of sediments or soil and does not move through the soil to underground water. If mercury enters the water in any form, it is likely to settle to the bottom where it can remain for a long time.
Mercury can enter and accumulate in the food chain. The form of mercury that accumulates in the food chain is methylmercury. Inorganic mercury does not accumulate up the food chain to any extent. When small fish eat the methylmercury in food, it goes into their tissues. When larger fish eat smaller fish or other organisms that contain methylmercury, most of the methylmercury originally present in the small fish will then be stored in the bodies of the larger fish. As a result, the larger and older fish living in contaminated waters build up the The levels of mercury in the atmosphere (i.e., the air you breathe in the general environment) are very, very low and do not pose a health risk. 8 highest amounts of methylmercury in their bodies. Saltwater fish (especially sharks and swordfish) that live a long time and can grow to a very large size tend to have the highest levels of mercury in their bodies. Plants (such as corn, wheat, and peas) have very low levels of mercury, even if grown in soils containing mercury at significantly higher than background levels. Mushrooms, however, can accumulate high levels if grown in contaminated soils.
Exposure to Mercury
 Because mercury occurs naturally in the environment, everyone is exposed to very low levels of mercury in air, water, and food.
Because mercury occurs naturally in the environment, everyone is exposed to very low levels of mercury in air, water, and food.
The levels of mercury that have been measured in urban outdoor air are hundreds of times lower than levels still considered to be “safe” to breathe. Background levels in nonurban settings are even lower. Mercury levels in surface water are generally about a thousand times lower than “safe” drinking water standards.
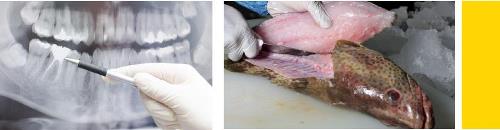
A potential source of exposure to metallic mercury for the general population is mercury released from dental amalgam fillings. An amalgam is a mixture of metals. The amalgam used in silver-colored dental fillings contains approximately 50% metallic mercury, 35% silver, 9% tin, 6% copper, and trace amounts of zinc. The mercury from dental amalgam may contribute from 0 to more than 75% of your total daily mercury exposure, depending on the number of amalgam fillings you have, the amount of fish consumed, the levels of mercury (mostly as methylmercury) in those fish, and exposure from other less common sources such as mercury spills, religious practices, or herbal remedies that contain mercury. However, it should be kept in mind that exposure to very small amounts of mercury, such as that from dental amalgam fillings, does not necessarily pose a health risk.
Whether the levels of exposure to mercury vapor from dental amalgam are sufficiently high to cause adverse health effects, and exactly what those effects are, continues to be researched and debated by scientists and health officials. U.S. government summaries on the effects of dental amalgam conclude that there is no apparent health hazard to the general population, but that further study is needed to determine the possibility of more subtle behavioral or immune system effects, and to determine the levels of exposure that may lead to adverse effects in sensitive populations. Sensitive populations may include pregnant women, children under the age of 6 (especially up to the age of 3), people with impaired kidney function, and people with hypersensitive immune responses to metals.
 Some religions have practices that may include the use of metallic mercury. Examples of these religions include Santeria (a Cuban-based religion whose followers worship both African deities and Catholic saints), Voodoo (a Haitian-based set of beliefs and rituals), Palo Mayombe (a secret form of ancestor worship practiced mainly in the Caribbean), and Espiritismo (a spiritual belief system native to Puerto Rico). Not all people who observe these religions use mercury, but when mercury is used in religious, ethnic, or ritualistic practices, exposure to mercury may occur both at the time of the practice and afterwards from contaminated indoor air. Metallic mercury is sold under the name “azogue” (pronounced ah-SEW-gay) in stores called “botanicas.” Botanicas are common in Hispanic and Haitian communities, where azogue may be sold as an herbal remedy or for spiritual practices. The metallic mercury is often sold in capsules or in glass containers. It may be placed in a sealed pouch to be worn on a necklace or in a pocket, or it may be sprinkled in the home or car. Some people may mix azogue in bath water or perfume, or place azogue in devotional candles. Because metallic mercury evaporates into the air, these practices may put anyone breathing the air in the room at risk of exposure to mercury. The longer people breathe the contaminated air, the greater their risk will be. The use of metallic mercury in a home or an apartment not only threatens the health of the people who live there now, but also threatens the health of future residents who may unknowingly be exposed to further release of mercury vapors from contaminated floors or walls.
Some religions have practices that may include the use of metallic mercury. Examples of these religions include Santeria (a Cuban-based religion whose followers worship both African deities and Catholic saints), Voodoo (a Haitian-based set of beliefs and rituals), Palo Mayombe (a secret form of ancestor worship practiced mainly in the Caribbean), and Espiritismo (a spiritual belief system native to Puerto Rico). Not all people who observe these religions use mercury, but when mercury is used in religious, ethnic, or ritualistic practices, exposure to mercury may occur both at the time of the practice and afterwards from contaminated indoor air. Metallic mercury is sold under the name “azogue” (pronounced ah-SEW-gay) in stores called “botanicas.” Botanicas are common in Hispanic and Haitian communities, where azogue may be sold as an herbal remedy or for spiritual practices. The metallic mercury is often sold in capsules or in glass containers. It may be placed in a sealed pouch to be worn on a necklace or in a pocket, or it may be sprinkled in the home or car. Some people may mix azogue in bath water or perfume, or place azogue in devotional candles. Because metallic mercury evaporates into the air, these practices may put anyone breathing the air in the room at risk of exposure to mercury. The longer people breathe the contaminated air, the greater their risk will be. The use of metallic mercury in a home or an apartment not only threatens the health of the people who live there now, but also threatens the health of future residents who may unknowingly be exposed to further release of mercury vapors from contaminated floors or walls.
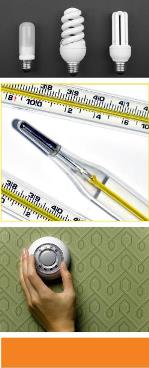
Metallic mercury is used in a variety of household products and industrial items, including thermostats, fluorescent light bulbs, barometers, glass thermometers, and some blood pressure devices. The mercury in these devices is contained in glass or metal, and generally does not pose a risk unless the item is damaged or broken, and mercury vapors are released. Spills of metallic mercury from broken thermometers or damaged electrical 11 switches in the home may result in exposure to mercury vapors in indoor air. You must be careful when you handle and dispose of all items in the home that contain metallic mercury.
 Very small amounts of metallic mercury (for example, a few drops) can raise air concentrations of mercury to levels that may be harmful to health. The longer people breathe the contaminated air, the greater the risk to their health. Metallic mercury and its vapors are extremely difficult to remove from clothes, furniture, carpet, floors, walls, and other such items. If these items are not properly cleaned, the mercury can remain for months or years, and continue to be a source of exposure.
Very small amounts of metallic mercury (for example, a few drops) can raise air concentrations of mercury to levels that may be harmful to health. The longer people breathe the contaminated air, the greater the risk to their health. Metallic mercury and its vapors are extremely difficult to remove from clothes, furniture, carpet, floors, walls, and other such items. If these items are not properly cleaned, the mercury can remain for months or years, and continue to be a source of exposure.
It is possible for you to be exposed to metallic mercury vapors from breathing contaminated air around hazardous waste sites, waste incinerators, or power plants that burn mercury-containing fuels (such as coal or other fossil fuels), but most outdoor air is not likely to contain levels that would be harmful. Exposure to mercury compounds at hazardous waste sites is much more likely to occur from handling contaminated soil (i.e., children playing in or eating contaminated surface soil), drinking well-water, or eating fish from contaminated waters near those sites. Not all hazardous sites contain mercury, and not all waste sites that do contain mercury have releases of mercury to the air, water, or surface soils.
You can be exposed to mercury vapors from the use of fungicides that contain mercury. Excess use of these products may result in higher-than-average exposures. You may also be exposed to mercury from swallowing or applying to your skin outdated medicinal products (laxatives, worming medications, and teething powders) that contain mercurous chloride. Exposure may also occur from the improper or excessive use of other chemicals containing mercury, such as skin-lightening creams and some topical antiseptic or disinfectant agents (mercurochrome and thimerosal).
 Workers are mostly exposed from breathing air that contains mercury vapors, but may also be exposed to other inorganic mercury compounds in the workplace. Occupations that have a greater potential for mercury exposure include manufacturers of electrical equipment or automotive parts that contain mercury, chemical processing plants that use mercury, metal processing, construction where building parts contain mercury (e.g., electrical switches, thermometers), and the medical professions (medical, dental, or other health services) where equipment may contain mercury (e.g., some devices that measure blood pressure contain liquid mercury). Dentists and their assistants may be exposed to metallic mercury from breathing in mercury vapor released from amalgam fillings and to a much lesser extent from skin contact with amalgam restorations. Family members of workers who have been exposed to mercury may also be exposed to mercury if the worker’s clothes are contaminated with mercury particles or liquid.
Workers are mostly exposed from breathing air that contains mercury vapors, but may also be exposed to other inorganic mercury compounds in the workplace. Occupations that have a greater potential for mercury exposure include manufacturers of electrical equipment or automotive parts that contain mercury, chemical processing plants that use mercury, metal processing, construction where building parts contain mercury (e.g., electrical switches, thermometers), and the medical professions (medical, dental, or other health services) where equipment may contain mercury (e.g., some devices that measure blood pressure contain liquid mercury). Dentists and their assistants may be exposed to metallic mercury from breathing in mercury vapor released from amalgam fillings and to a much lesser extent from skin contact with amalgam restorations. Family members of workers who have been exposed to mercury may also be exposed to mercury if the worker’s clothes are contaminated with mercury particles or liquid.
Some people may be exposed to higher levels of mercury in the form of methylmercury if they have a diet high in fish, shellfish, or marine mammals (whales, seals, dolphins, and walruses) that come from mercury-contaminated waters. Methylmercury accumulates up the food chain, so that fish at the top of the food chain will have the most mercury in their flesh. Of these fish, the largest (i.e., the oldest) fish will have the highest levels. Commercial fish sold through interstate commerce that are found to have levels of methylmercury above an “action level” of 1 ppm (established by the FDA) cannot be sold to the public. This level itself is below a level associated with adverse effects. Public health advisories are issued by state and federal authorities for local waters that are thought to be contaminated with mercury. Foods other than fish that may contain higher than average levels of mercury include wild game, such as wild birds and mammals (bear) that eat large amounts of contaminated fish. People in the most northern climates may be exposed to high levels of mercury from eating meat or fat from marine mammals including whales, dolphins, walruses, and seals. Plants contain very little methylmercury or other forms of mercury. Mushrooms grown in mercury-contaminated soil may contain levels of mercury that could pose some risk to health, if large amounts were eaten.
Mercury and the Body
 Oral exposure to mercury results in very small amounts getting into your body.
Oral exposure to mercury results in very small amounts getting into your body.
When you swallow small amounts of metallic mercury, for example, from a broken oral thermometer, virtually none (less than 0.01%) of the mercury will enter your body through the stomach or intestines, unless they are diseased. Even when a larger amount of metal mercury (a half of a tablespoon, about 204 grams) was swallowed by one person, very little entered the body. When you breathe in mercury vapors, however, most (about 80%) of the mercury enters your bloodstream directly from your lungs, and then rapidly goes to other parts of your body, including the brain and kidneys. Once in your body, metallic mercury can stay for weeks or months. When metallic mercury enters the brain, it is readily converted to an inorganic form and is “trapped” in the brain for a long time. Metallic mercury in the blood of a pregnant woman can enter her developing child. Most of the metallic mercury will accumulate in your kidneys, but some metallic mercury can also accumulate in the brain. Most of the metallic mercury absorbed into the body eventually leaves in the urine and feces, while smaller amounts leave the body in the exhaled breath.
 Inorganic mercury compounds like mercurous chloride and mercuric chloride are white powders and do not generally vaporize at room temperatures like elemental mercury will. If they are inhaled, they are not expected to enter your body as easily as inhaled metallic mercury vapor. When inorganic mercury compounds are swallowed, generally less than 10% is absorbed through the intestinal tract; however, up to 40% may enter the body through the stomach and intestines in some instances. Some inorganic mercury can enter your body through the skin, but only a small amount will pass through your skin compared to the amount that gets into your body from swallowing inorganic mercury. Once inorganic mercury enters the body and gets into the bloodstream, it moves to many different tissues. Inorganic mercury leaves your body in the urine or feces over a period of several weeks or months. A small amount of the inorganic mercury can be changed in your body to metallic mercury and leave in the breath as a mercury vapor. Inorganic mercury accumulates mostly in the kidneys and does not enter the brain as easily as metallic mercury. Inorganic mercury compounds also do not move as easily from the blood of a pregnant woman to her developing child. In a nursing woman, some of the inorganic mercury in her body will pass into her breast milk.
Inorganic mercury compounds like mercurous chloride and mercuric chloride are white powders and do not generally vaporize at room temperatures like elemental mercury will. If they are inhaled, they are not expected to enter your body as easily as inhaled metallic mercury vapor. When inorganic mercury compounds are swallowed, generally less than 10% is absorbed through the intestinal tract; however, up to 40% may enter the body through the stomach and intestines in some instances. Some inorganic mercury can enter your body through the skin, but only a small amount will pass through your skin compared to the amount that gets into your body from swallowing inorganic mercury. Once inorganic mercury enters the body and gets into the bloodstream, it moves to many different tissues. Inorganic mercury leaves your body in the urine or feces over a period of several weeks or months. A small amount of the inorganic mercury can be changed in your body to metallic mercury and leave in the breath as a mercury vapor. Inorganic mercury accumulates mostly in the kidneys and does not enter the brain as easily as metallic mercury. Inorganic mercury compounds also do not move as easily from the blood of a pregnant woman to her developing child. In a nursing woman, some of the inorganic mercury in her body will pass into her breast milk.
Methylmercury is the form of mercury most easily absorbed through the gastrointestinal tract (about 95% absorbed). After you eat fish or other foods that are contaminated with methylmercury, the methylmercury enters your bloodstream easily and goes rapidly to other parts of your body. Only small amounts of methylmercury enter the bloodstream directly through the skin, but other forms of organic mercury (in particular dimethylmercury) can rapidly enter the body through the skin. Organic mercury compounds may evaporate slowly at room temperature and may enter your body easily if you breathe in the vapors. Once organic mercury is in the bloodstream, it moves easily to most tissues and readily enters the brain. Methylmercury that is in the blood of a pregnant woman will easily move into the blood of the developing child and then into the child’s brain and other tissues. Like metallic mercury, methylmercury can be changed by your body to inorganic mercury. When this happens in the brain, the mercury can remain there for a long time. When methylmercury does leave your body after you have been exposed, it leaves slowly over a period of several months, mostly as inorganic mercury in the feces. As with inorganic mercury, some of the methylmercury in a nursing woman’s body will pass into her breast milk. Top of Page
Health Effects of Exposure
 The nervous system is very sensitive to mercury.
The nervous system is very sensitive to mercury.
In poisoning incidents that occurred in other countries, some people who ate fish contaminated with large amounts of methylmercury or seed grains treated with methylmercury or other organic mercury compounds developed permanent damage to the brain and kidneys. Permanent damage to the brain has also been shown to occur from exposure to sufficiently high levels of metallic mercury. Whether exposure to inorganic mercury results in brain or nerve damage is not as certain, since it does not easily pass from the blood into the brain.
Metallic mercury vapors or organic mercury may affect many different areas of the brain and their associated functions, resulting in a variety of symptoms. These include personality changes (irritability, shyness, nervousness), tremors, changes in vision (constriction (or narrowing) of the visual field), deafness, muscle incoordination, loss of sensation, and difficulties with memory.
Different forms of mercury have different effects on the nervous system, because they do not all move through the body in the same way. When metallic mercury vapors are inhaled, they readily enter the bloodstream and are carried throughout the body and can move into the brain. Breathing in or swallowing large amounts of methylmercury also results in some of the mercury moving into the brain and affecting the nervous system. Inorganic mercury salts, such as mercuric chloride, do not enter the brain as readily as methylmercury or metallic mercury vapor.
The kidneys are also sensitive to the effects of mercury, because mercury accumulates in the kidneys and causes higher exposures to these tissues, and thus more damage. All forms of mercury can cause kidney damage if large enough amounts enter the body. If the damage caused by the mercury is not too great, the kidneys are likely to recover once the body clears itself of the contamination.

Short-term exposure (hours) to high levels of metallic mercury vapor in the air can damage the lining of the mouth and irritate the lungs and airways, causing tightness of the breath, a burning sensation in the lungs, and coughing. Other effects from exposure to mercury vapor include nausea, vomiting, diarrhea, increases in blood pressure or heart rate, skin rashes, and eye irritation. Damage to the lining of the mouth and lungs can also occur from exposure to lower levels of mercury vapor over longer periods (for example, in some occupations where workers were exposed to mercury for many years). Levels of metallic mercury in workplace air are generally much greater than the levels normally encountered by the general population. Current levels of mercury in workplace air are low, due to increased awareness of mercury’s toxic effects. Because of the reduction in the allowable amount of mercury in workplace air, fewer workers are expected to have symptoms of mercury toxicity. Most studies of humans who breathed metallic mercury for a long time indicate that mercury from this type of exposure does not affect the ability to have children. Studies in workers exposed to metallic mercury vapors have also not shown any mercury-related increase in cancer. Skin contact with metallic mercury has been shown to cause an allergic reaction (skin rashes) in some people.
In addition to effects on the kidneys, inorganic mercury can damage the stomach and intestines, producing symptoms of nausea, diarrhea, or severe ulcers if swallowed in large amounts. Effects on the heart have also been observed in children after they accidentally swallowed mercuric chloride. Symptoms included rapid heart rate and increased blood pressure. There is little information on the effects in humans from long-term, low-level exposure to inorganic mercury. Studies using animals indicate that long-term oral exposure to inorganic mercury salts causes kidney damage, effects on blood pressure and heart rate, and effects on the stomach. Some rat and mice strains that are susceptible to autoimmune responses develop kidney damage as a result of an immune response when exposed to relatively low levels of mercury vapor or mercury chloride.
Mercury and Children
Children are at risk of being exposed to mercury in a number of ways.
 Some of these routes may include exposure to metallic mercury that is not safely contained, to mercury that may be brought home on work clothes or tools, or to methylmercury-contaminated foods. Methylmercury eaten or swallowed by a pregnant woman or metallic mercury that enters her body from breathing contaminated air can also pass into the developing child. Inorganic mercury and methylmercury can also pass from a mother’s body into breast milk and into a nursing infant. The amount of mercury in the milk will vary, depending on the degree of exposure and the amount of mercury that enter the nursing woman’s body. There are significant benefits to breast feeding, so any concern that a nursing woman may have about mercury levels in her breast milk should be discussed with her doctor. Methylmercury can also accumulate in an unborn baby’s blood to a concentration higher than the concentration in the mother.
Some of these routes may include exposure to metallic mercury that is not safely contained, to mercury that may be brought home on work clothes or tools, or to methylmercury-contaminated foods. Methylmercury eaten or swallowed by a pregnant woman or metallic mercury that enters her body from breathing contaminated air can also pass into the developing child. Inorganic mercury and methylmercury can also pass from a mother’s body into breast milk and into a nursing infant. The amount of mercury in the milk will vary, depending on the degree of exposure and the amount of mercury that enter the nursing woman’s body. There are significant benefits to breast feeding, so any concern that a nursing woman may have about mercury levels in her breast milk should be discussed with her doctor. Methylmercury can also accumulate in an unborn baby’s blood to a concentration higher than the concentration in the mother.
For similar exposure routes and forms of mercury, the harmful health effects seen in children are similar to the effects seen in adults. High exposure to mercury vapor causes lung, stomach, and intestinal damage and death due to respiratory failure in severe cases. These effects are similar to those seen in adult groups exposed to inhaled metallic mercury vapors at work.
Children who had been exposed to excessive amounts of mercurous chloride tablets for worms or mercurous chloride-containing powders for teething discomfort had increased heart rates and elevated blood pressure. Abnormal heart rhythms were also seen in children who had eaten grains contaminated with very high levels of methylmercury.
 Other symptoms of poisonings in children who were treated with mercurous chloride for constipation, worms, or teething discomfort included swollen red gums, excessive salivation, weight loss, diarrhea and/or abdominal pain, and muscle twitching or cramping in the legs and/or arms. Kidney damage is very common after exposure to toxic levels of inorganic mercury. Metallic mercury or methylmercury that enters the body can also be converted to inorganic mercury and result in kidney damage. Children who breathe metallic/elemental mercury vapors, eat foods or other substances containing phenylmercury or inorganic mercury salts, or use mercurycontaining skin ointments for an extended period may develop a disorder known as acrodynia, or pink disease. Acrodynia can result in severe leg cramps; irritability; and abnormal redness of the skin, followed by peeling of the hands, nose, and soles of the feet. Itching, swelling, fever, fast heart rate, elevated blood pressure, excessive salivation or sweating, rashes, fretfulness, sleeplessness, and/or weakness may also be present. It was once believed that this syndrome occurred only in children, but recent reported cases in teenagers and adults have shown that they can also develop acrodynia.
Other symptoms of poisonings in children who were treated with mercurous chloride for constipation, worms, or teething discomfort included swollen red gums, excessive salivation, weight loss, diarrhea and/or abdominal pain, and muscle twitching or cramping in the legs and/or arms. Kidney damage is very common after exposure to toxic levels of inorganic mercury. Metallic mercury or methylmercury that enters the body can also be converted to inorganic mercury and result in kidney damage. Children who breathe metallic/elemental mercury vapors, eat foods or other substances containing phenylmercury or inorganic mercury salts, or use mercurycontaining skin ointments for an extended period may develop a disorder known as acrodynia, or pink disease. Acrodynia can result in severe leg cramps; irritability; and abnormal redness of the skin, followed by peeling of the hands, nose, and soles of the feet. Itching, swelling, fever, fast heart rate, elevated blood pressure, excessive salivation or sweating, rashes, fretfulness, sleeplessness, and/or weakness may also be present. It was once believed that this syndrome occurred only in children, but recent reported cases in teenagers and adults have shown that they can also develop acrodynia.
In critical periods of development before they are born, and in the early months after birth, children and fetuses are particularly sensitive to the harmful effects of metallic mercury and methylmercury on the nervous system. Harmful developmental effects may occur when a pregnant woman is exposed to metallic mercury and some of the mercury is transferred into her developing child. Thus, women who are normally exposed to mercury vapors in the workplace (such as those working in thermometer/barometer or fluorescent light manufacturing or the chloralkali industry) should take measures to avoid mercury vapor exposures during pregnancy. Exposures to mercury vapors are relatively rare outside of the workplace, unless metallic mercury is present in the home.
As with mercury vapors, exposure to methylmercury is more dangerous for young children than for adults, because more methylmercury easily passes into the developing brain of young children and may interfere with the development process.
Methylmercury is the form of mercury most commonly associated with a risk for developmental effects. Exposure can come from foods contaminated with mercury on the surface (for example, from seed grain treated with methylmercury to kill fungus) or from foods that contain toxic levels of methylmercury (as in some fish, wild game, and marine mammals). Mothers who are exposed to methylmercury and breast-feed their infant may also expose the child through the milk. The effects on the infant may be subtle or more pronounced, depending on the amount to which the fetus or young child was exposed. In cases in which the exposure was very small, some effects might not be apparent, such as small decreases in IQ or effects on the brain that may only be determined by the use of very sensitive neuropsychological testing. In instances in which the exposure is great, the effects may be more serious. In some such cases of mercury exposure involving serious exposure to the developing fetus, the effects are delayed. In such cases, the infant may be born apparently normal, but later show effects that may range from the infant being slower to reach developmental milestones, such as the age of first walking and talking, to more severe effects including brain damage with mental retardation, incoordination, and inability to move.

Other severe effects observed in children whose mothers were exposed to very toxic levels of mercury during pregnancy include eventual blindness, involuntary muscle contractions and seizures, muscle weakness, and inability to speak. It is important to remember, however, that the severity of these effects depends upon the level of mercury exposure and the time of exposure. The very severe effects just mentioned were reported in large-scale poisoning instances in which pregnant and nursing women were exposed to extremely high levels of methylmercury in contaminated grain used to make bread (in Iraq) or seafood (in Japan) sold to the general population.
Researchers are currently studying the potential for less serious developmental effects, including effects on a child’s behavior and ability to learn, think, and solve problems that may result from eating lower levels of methylmercury in foods. A main source of exposure to methylmercury for the pregnant woman and the young child is from eating fish. Most fish purchased in the market in the United States do not have mercury levels that pose a risk to anyone, including pregnant women. Since mercury accumulates in the muscles of fish, larger fish that feed on smaller fish and live for long periods usually have larger concentrations of methylmercury than fish that feed on plants. For example, shark and swordfish normally contain the highest levels of mercury out of all ocean fish. Scientists have an ongoing debate about the value of fish in the diet versus any risk from increased exposure of pregnant women to methylmercury that may be in the fish. The safety of most fish sold commercially in the United States is regulated by the FDA. These fish pose no health risk to those who purchase and eat them. Only fish or wildlife containing relatively high levels of methylmercury are of concern. Top of Page
Reducing Risk of Exposure
 If your doctor finds that you have been exposed to significant amounts of mercury, ask whether your children might also be exposed. Your doctor might need to ask your state health department to investigate.
If your doctor finds that you have been exposed to significant amounts of mercury, ask whether your children might also be exposed. Your doctor might need to ask your state health department to investigate.
Children may be exposed to metallic mercury if they play with it. Metallic mercury is a heavy, shiny, silver liquid. When metallic mercury is spilled, it forms little balls or beads. Children are sometimes exposed to metallic mercury when they find it in abandoned warehouses or closed factories, and then play with it or pass it around to friends. Children have also taken metallic mercury from school chemistry and physics labs. Broken thermometers and some electrical switches are other sources of metallic mercury. Sometimes children find containers of metallic mercury that were improperly disposed of, or adults may bring home metallic mercury from work, not knowing that it is dangerous.
To protect your children from metallic mercury, teach them not to play with shiny, silver liquids. Schoolteachers (particularly science teachers) and school staff need to know about students’ fascination with metallic mercury. Teachers and school staff should teach children about the dangers of getting sick from playing with mercury, and they should keep metallic mercury in a safe and secured area (such as a closed container in a locked storage room) so that children do not have access to it without the supervision of a teacher. Metallic mercury evaporates slowly, and if it is not stored in a closed container, children may breathe toxic mercury vapors.
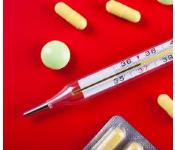 In the past, mercurous chloride was widely used in medicinal products such as laxatives, worming medications, and teething powders. These older medicines should be properly disposed of and replaced with safer and more effective medicines. Other chemicals containing mercury, such as mercurochrome and thimerosal (sold as Merthiolate and other brands), are still used as antiseptics or as preservatives in eye drops, eye ointments, nasal sprays, and vaccines. Some skin-lightening creams contain ammoniated mercuric chloride and mercuric iodide. These and all other mercury-containing medicines should be kept safely out of the reach of children to prevent an accidental poisoning. Nonmedicinal products, including some fungicides that contain mercury compounds and paints that contain mercuric sulfide or mercuric oxide, should also be safely stored out of the reach of children.
In the past, mercurous chloride was widely used in medicinal products such as laxatives, worming medications, and teething powders. These older medicines should be properly disposed of and replaced with safer and more effective medicines. Other chemicals containing mercury, such as mercurochrome and thimerosal (sold as Merthiolate and other brands), are still used as antiseptics or as preservatives in eye drops, eye ointments, nasal sprays, and vaccines. Some skin-lightening creams contain ammoniated mercuric chloride and mercuric iodide. These and all other mercury-containing medicines should be kept safely out of the reach of children to prevent an accidental poisoning. Nonmedicinal products, including some fungicides that contain mercury compounds and paints that contain mercuric sulfide or mercuric oxide, should also be safely stored out of the reach of children.
You should check to see if any medicines or herbal remedies that you or your child use contain mercury. Some traditional Chinese and Indian remedies for stomach disorders (for example, herbal balls) contain mercury, and if you give these remedies to your children, you may harm them. If you are pregnant or nursing a baby and you use mercury-containing ethnic or herbal remedies, you could pass some of the mercury to your unborn child or nursing infant.
If you use metallic mercury or azogue in religious practices, you may expose your children or unborn child to mercury or contaminate your home. Such practices in which mercury containing substances have traditionally been used include Santeria (a Cuban-based religion whose followers worship both African deities and Catholic saints), Voodoo (a Haitianbased set of beliefs and rituals), Palo Mayombe (a secret form of ancestor worship practiced mainly in the Caribbean), or Espiritismo (a spiritual belief system native to Puerto Rico).
 Metallic mercury is used in a variety of household products and industrial items, including thermostats, fluorescent light bulbs, barometers, glass thermometers, and some blood pressure measuring devices. You must be careful when you handle and dispose of all items in the home that contain metallic mercury.
Metallic mercury is used in a variety of household products and industrial items, including thermostats, fluorescent light bulbs, barometers, glass thermometers, and some blood pressure measuring devices. You must be careful when you handle and dispose of all items in the home that contain metallic mercury.
If small amounts of mercury are spilled, be very careful cleaning it up. Do not try to vacuum spilled metallic mercury. You should check to see if any medicines or herbal remedies that you or your child use contain mercury. 24 Using a vacuum cleaner to clean up the mercury causes the mercury to evaporate into the air, creating greater health risks. Trying to vacuum spilled metallic mercury also contaminates the vacuum cleaner. Also, take care not to step on the mercury and track it into other areas of the home. Metallic mercury vapors are very toxic and have no odor. Do not remain unnecessarily in that room, and try not to let metallic mercury contact your eyes, skin, or clothing. If you think you have been exposed directly to metallic mercury, wash yourself thoroughly and discard contaminated clothing by placing them in a sealed plastic bag. Perhaps the most important thing to remember if you break a household thermometer is do not panic. The amount of mercury contained in an oral thermometer is small and does not present an immediate threat to human health. However, if it is not properly cleaned up and disposed of, it may present a health risk over time, particularly to infants, toddlers, and pregnant women.
 If a thermometer breaks on a counter top or uncarpeted floor, remove children from the area. Mercury is not absorbent, so do not try to wipe or blot it up with a cloth or paper towel; that will only spread the mercury and break it up into smaller beads, making it more difficult to find and remove. Instead, clean up the beads of metallic mercury by using one sheet of paper to carefully roll them onto a second sheet of paper, or by sucking very small beads of mercury into an eye dropper. After picking up the metallic mercury in this manner, put it into a plastic bag or airtight container. The paper and eye dropper should also be bagged in a zip-lock plastic container. All plastic bags used in the cleanup should then be taken outside of the house or apartment and disposed of properly, according to instructions provided by your local health department or environmental officials. Try to ventilate the room with outside air, and close the room off from the rest of the home. Use fans (that direct the air to the outside and away from the inside of the house) for a minimum of one hour to speed the ventilation.
If a thermometer breaks on a counter top or uncarpeted floor, remove children from the area. Mercury is not absorbent, so do not try to wipe or blot it up with a cloth or paper towel; that will only spread the mercury and break it up into smaller beads, making it more difficult to find and remove. Instead, clean up the beads of metallic mercury by using one sheet of paper to carefully roll them onto a second sheet of paper, or by sucking very small beads of mercury into an eye dropper. After picking up the metallic mercury in this manner, put it into a plastic bag or airtight container. The paper and eye dropper should also be bagged in a zip-lock plastic container. All plastic bags used in the cleanup should then be taken outside of the house or apartment and disposed of properly, according to instructions provided by your local health department or environmental officials. Try to ventilate the room with outside air, and close the room off from the rest of the home. Use fans (that direct the air to the outside and away from the inside of the house) for a minimum of one hour to speed the ventilation.
If a thermometer breaks and the liquid/metallic mercury spills onto a carpeted floor, try to collect the mercury beads in the manner described in the above paragraph. Do not try to vacuum spilled metallic mercury. Using a vacuum cleaner to clean up the mercury causes the mercury to evaporate into the air, creating greater health risks. 25 Depending on the cut or pile of the carpeting, however, it may not be possible to collect all of the spilled mercury. Regardless, do not vacuum. Instead, call your local (county, city, or state) health department and tell them of your situation. (You may also call the Agency for Toxic Substances and Disease Registry [ATSDR] toll-free at 1-888-42-ATSDR [1-888-422-8737] to obtain additional guidance, if local assistance cannot be obtained.)
If larger amounts of metallic mercury are found (for example, a jar of liquid mercury), it should be contained in an airtight container, and you should call your local health department for instructions on how to safely dispose of it. If the mercury is in an open container or the container does not have a lid, place a piece of plastic wrap around the top of the container to prevent vapors from escaping; then wash your hands thoroughly. If a larger amount is spilled, leave the area and contact your local health department and fire department. Do not simply throw metallic mercury away, but instead seek professional help.
ATSDR and EPA strongly recommend against the use of metallic (liquid) mercury that is not properly enclosed in glass, as it is in thermometers. This form of mercury should not be used or stored in homes, automobiles, day-care centers, schools, offices, or other public buildings. If you notice a child with metallic mercury on his or her clothing, skin, or hair, call the fire department and let them know that the child needs to be decontaminated.
 Metallic or inorganic mercury can be carried into the home from a workers’ contaminated clothing and shoes. Increased exposure to mercury has been reported in children of workers who are exposed to mercury at work, and increased levels of mercury were measured in places where work clothes were stored and in some washing machines. The children most likely to be exposed to risky levels of mercury are those whose parents work in facilities that use mercury (for example, a scientific glassware manufacturing plant or a chlor-alkali chemical plant), but where no protective uniforms or footgear are used. In some reported cases in which children were exposed in this way, protective clothing was used in the workplace by the parent, but work gloves, clothes, and boots, which were contaminated with mercury, were taken home, thus exposing family members.
Metallic or inorganic mercury can be carried into the home from a workers’ contaminated clothing and shoes. Increased exposure to mercury has been reported in children of workers who are exposed to mercury at work, and increased levels of mercury were measured in places where work clothes were stored and in some washing machines. The children most likely to be exposed to risky levels of mercury are those whose parents work in facilities that use mercury (for example, a scientific glassware manufacturing plant or a chlor-alkali chemical plant), but where no protective uniforms or footgear are used. In some reported cases in which children were exposed in this way, protective clothing was used in the workplace by the parent, but work gloves, clothes, and boots, which were contaminated with mercury, were taken home, thus exposing family members.
The Occupational Safety and Health Administration (OSHA) requires employers to provide Material Safety Data Sheets (MSDSs) for many of the chemicals used at the workplace. Information on these sheets should include chemical names and hazardous ingredients, important properties (such as fire and explosion data), potential health effects, how you get the chemical(s) in your body, how to properly handle the materials, and what to do in an emergency. Your occupational health and safety officer at work can and should tell you whether chemicals you work with are dangerous and likely to be carried home on your clothes, body, or tools, and whether you should be showering and changing clothes before you leave work, storing your street clothes in a separate area of the workplace, or laundering your work clothes at home separately from other clothes. Your employer is legally responsible for providing a safe workplace and should freely answer your questions about hazardous chemicals. Your OSHA-approved state occupational safety and health program or OSHA can also answer any further questions you might have, and help your employer identify and correct problems with hazardous substances. If you would like to make a formal complaint about health hazards in your workplace, your OSHAapproved state occupational safety and health program or OSHA office will listen to your complaint and inspect your workplace when necessary.
If you are pregnant, the decision of whether to have dental amalgam or a nonmercury material used for fillings, or whether existing amalgam fillings should be repaired or replaced during pregnancy, should be made in consultation with your dentist. The practice of having all your dental amalgam fillings replaced with non-mercury filling materials just to remove the possibility of mercury exposure is not recommended by ATSDR. In fact, the removal of the mercury amalgam fillings would actually expose the patient to a greater amount of mercury for a while.
 FDA currently advises that pregnant women and women of childbearing age who may become pregnant limit their consumption of shark and swordfish to no more that one meal per month. This advice is given because methylmercury levels are relatively high in these fish species. Women of childbearing age are included in this advice because dietary practices immediately before the pregnancy could have a direct bearing on fetal exposure during pregnancy, particularly during the earlier months of pregnancy.
FDA currently advises that pregnant women and women of childbearing age who may become pregnant limit their consumption of shark and swordfish to no more that one meal per month. This advice is given because methylmercury levels are relatively high in these fish species. Women of childbearing age are included in this advice because dietary practices immediately before the pregnancy could have a direct bearing on fetal exposure during pregnancy, particularly during the earlier months of pregnancy.
FDA further advises that persons other than pregnant women and women of childbearing age in the general population limit their regular consumption of shark and swordfish (which typically contains methylmercury around 1 ppm) to about 7 ounces per week (about one serving) to stay below the acceptable daily intake for methylmercury. For fish species with methylmercury levels averaging 0.5 ppm, regular consumption should be limited to 14 ounces per week. Recreational and subsistence fishers who eat larger amounts of fish than the general population and routinely fish the same waterbodies may have a higher exposure to methylmercury if these waters are contaminated. People who consume greater than 100 grams of fish (approximately 3.5 ounces) every day are considered high-end consumers. This is over 10 times more than the amount of fish consumed by members of the general population (6.5 g/day). No consumption advice is necessary for the top ten seafood species that make up about 80% of the seafood sold in the United States: canned tuna, shrimp, pollock, salmon, cod, catfish, clams, flatfish, crabs, and scallops. The methylmercury in these species is generally less than 0.2 ppm, and few people eat more than the suggested weekly limit of fish (i.e., 2.2 pounds). Top of Page
Exposure Testing
 There are reliable and accurate ways to measure mercury levels in the body.
There are reliable and accurate ways to measure mercury levels in the body.
These tests all involve taking blood, urine, or hair samples, and must be performed in a doctor’s office or in a health clinic. Nursing women may have their breast milk tested for mercury levels, if any of the other samples tested are found to contain significant amounts of mercury. Most of these tests, however, do not determine the form of mercury to which you were exposed. Mercury levels found in blood, urine, breast milk, or hair may be used to determine if adverse health effects are likely to occur. Mercury in urine is used to test for exposure to metallic mercury vapor and to inorganic forms of mercury. Measurement of mercury in whole blood or scalp hair is used to monitor exposure to methylmercury. Urine is not useful for determining whether exposure has occurred to methylmercury. Levels found in blood, urine, and hair may be used together to predict possible health effects that may be caused by the different forms of mercury.
 Blood and urine levels are used as markers to determine whether someone has been exposed to mercury. They are used to determine whether exposure to mercury has occurred and to give a rough idea of the extent of exposure, but they do not tell exactly how much exposure has occurred. Except for methylmercury exposures, blood is considered useful if samples are taken within a few days of exposure. This is because most forms of mercury in the blood decrease by one-half every three days if exposure has been stopped. Thus, mercury levels in the blood provide more useful information after recent exposures than after long-term exposures. Several months after an exposure, mercury levels in the blood and urine are much lower. Hair, which is considered useful only for exposures to methylmercury, can be used to show exposures that occurred many months ago, or even more than a year ago if the hair is long enough and careful testing. Top of Page
Blood and urine levels are used as markers to determine whether someone has been exposed to mercury. They are used to determine whether exposure to mercury has occurred and to give a rough idea of the extent of exposure, but they do not tell exactly how much exposure has occurred. Except for methylmercury exposures, blood is considered useful if samples are taken within a few days of exposure. This is because most forms of mercury in the blood decrease by one-half every three days if exposure has been stopped. Thus, mercury levels in the blood provide more useful information after recent exposures than after long-term exposures. Several months after an exposure, mercury levels in the blood and urine are much lower. Hair, which is considered useful only for exposures to methylmercury, can be used to show exposures that occurred many months ago, or even more than a year ago if the hair is long enough and careful testing. Top of Page
Governmental Recommendations
For the most current information, check with the federal agency or organization that provides it for the substance in which you are interested.
Some regulations and recommendations for mercury include the following:
EPA and FDA have set a limit of 2 parts inorganic mercury per billion (ppb) parts of water in drinking water. EPA is in the process of revising the Water Quality Criteria for mercury.
EPA currently recommends that the level of inorganic mercury in rivers, lakes, and streams be no more than 144 parts mercury per trillion (ppt) parts of water to protect human health (1 ppt is a thousand times less than 1 part per billion, or ppb).
EPA has determined that a daily exposure (for an adult of average weight) to inorganic mercury in drinking water at a level up to 2 ppb is not likely to cause any significant adverse health effects.
FDA has set a maximum permissible level of 1 part of methylmercury in a million parts (ppm) of seafood products sold through interstate commerce (1 ppm is a thousand times more than 1 ppb). FDA may seize shipments of fish and shellfish containing more than 1 ppm of methylmercury, and may seize treated seed grain containing more than 1 ppm of mercury.
OSHA has set a limit of 0.1 milligrams of mercury per cubic meter of air (mg/m3) for aryl mercury and 0.01 mg/m3 for alkyl mercury to protect workers during an 8-hour shift and a 40-hour work week. NIOSH recommends that the amount of metallic mercury vapor in workplace air be limited to an average level of 0.05 mg/m3 during a 10-hour work shift.
Removal of dental amalgams in people who have no indication of adverse effects is not recommended and can put the person at greater risk, if performed improperly. Chelation therapy (used to remove metals from the body tissues) itself presents some health risks, and should be considered only when a licensed occupational or environmental health physician determines it necessary to reduce immediate and significant health risks due to high levels of mercury in the body. Top of Page
Additional Information
If you have more questions or concerns, please contact your community or state health or environmental quality department, or contact ATSDR at the address and phone number below.
ATSDR also can tell you the location of occupational and environmental health clinics. These clinics specialize in recognizing, evaluating, and treating illnesses that result from exposure to hazardous substances.
Agency for Toxic Substances and Disease Registry
Division of Toxicology and Human Health Sciences
1600 Clifton Road NE,
Mailstop F-57
Atlanta, GA 30333
Toll-free information and technical assistance: 1-800-CDCINFO (1-800-232-4636)
For additional information, please check out ATSDR’s Don’t Mess with Mercury website. Top of Page

- Page last reviewed: October 14, 2015
- Page last updated: October 14, 2015
- Content source:



 ShareCompartir
ShareCompartir