Arsenic
Arsenic Overview
Arsenic is a naturally occurring element that is widely distributed in the Earth’s crust.
 Arsenic is classified chemically as a metalloid, having both properties of a metal and a nonmetal; however, it is frequently referred to as a metal. Elemental arsenic (sometimes referred to as metallic arsenic) is a steel grey solid material. However, arsenic is usually found in the environment combined with other elements such as oxygen, chlorine, and sulfur. Arsenic combined with these elements is called inorganic arsenic. Arsenic combined with carbon and hydrogen is referred to as organic arsenic.
Arsenic is classified chemically as a metalloid, having both properties of a metal and a nonmetal; however, it is frequently referred to as a metal. Elemental arsenic (sometimes referred to as metallic arsenic) is a steel grey solid material. However, arsenic is usually found in the environment combined with other elements such as oxygen, chlorine, and sulfur. Arsenic combined with these elements is called inorganic arsenic. Arsenic combined with carbon and hydrogen is referred to as organic arsenic.
Most inorganic and organic arsenic compounds are white or colorless powders that do not evaporate. They have no smell, and most have no special taste. Thus, you usually cannot tell if arsenic is present in your food, water, or air.
 Inorganic arsenic occurs naturally in soil and in many kinds of rock. When these ores are heated in smelters, most of the arsenic goes up the stack and enters the air as a fine dust. Smelters may collect this dust and take out the arsenic as a compound called arsenic trioxide (As2O3). However, arsenic is no longer produced in the United States; all of the arsenic used in the United States is imported.
Inorganic arsenic occurs naturally in soil and in many kinds of rock. When these ores are heated in smelters, most of the arsenic goes up the stack and enters the air as a fine dust. Smelters may collect this dust and take out the arsenic as a compound called arsenic trioxide (As2O3). However, arsenic is no longer produced in the United States; all of the arsenic used in the United States is imported.
Presently, about 90% of all arsenic produced is used as a preservative for wood to make it resistant to rotting, attack by termites and fungi, and decay. The preservative is copper chromated arsenate (CCA)—a water-soluble inorganic pesticide—and the treated wood is referred to as “pressure-treated.” In 2003, U.S. manufacturers of wood preservatives containing arsenic began a voluntary transition from CCA to other wood preservatives that do not contain arsenic for certain residential uses, such as play structures, picnic tables, decks, fencing, and boardwalks. This phase out was completed on December 31, 2003; however, wood treated prior to this date could still be used and existing structures made with CCA-treated wood would not be affected. CCA-treated wood products continue to be used in industrial applications. It is not known whether, or to what extent, CCA-treated wood products may contribute to exposure of people to arsenic.

In the past, inorganic arsenic compounds were predominantly used as pesticides, primarily on cotton fields and in orchards. Inorganic arsenic compounds can no longer be used in agriculture. However, organic arsenic compounds, namely cacodylic acid, disodium methylarsenate (DSMA), and monosodium methylarsenate (MSMA), are still used as pesticides, principally on cotton. Some organic arsenic compounds are used as additives in animal feed. Small quantities of elemental arsenic are added to other metals to form metal mixtures or alloys with improved properties. The greatest use of arsenic in alloys is in lead-acid batteries for automobiles. Another important use of arsenic compounds is in semiconductors and light-emitting diodes. Top of Page
Arsenic and the Environment
Arsenic occurs naturally in soil and minerals and it therefore may enter the air, water, and land from wind-blown dust and may get into water from runoff and leaching.
Volcanic eruptions are another natural source of arsenic. Arsenic is associated with ores containing metals, such as copper and lead. Arsenic may enter the environment during the mining and smelting of these ores. Small amounts of arsenic also may be released into the atmosphere from coal-fired power plants and incinerators because coal and waste products often contain some arsenic.

Arsenic cannot be destroyed in the environment. It can only change its form, or become attached to or separated from particles. It may change its form by reacting with oxygen or other molecules present in air, water, or soil, or by the action of bacteria that live in soil or sediment. Arsenic released from power plants and other combustion processes is usually attached to very small particles. Arsenic contained in wind-borne soil is generally found in larger particles. These particles settle to the ground or are washed out of the air by rain.
 Arsenic that is attached to very small particles may stay in the air for many days and travel long distances. Many common arsenic compounds can dissolve in water. Thus, arsenic can get into lakes, rivers, or underground water by dissolving in rain or snow or through the discharge of industrial wastes. Some of the arsenic will stick to particles in the water or sediment on the bottom of lakes or rivers, and some will be carried along by the water. Ultimately, most arsenic ends up in the soil or sediment. Although some fish and shellfish take in arsenic, which may build up in tissues, most of this arsenic is in an organic form called arsenobetaine (commonly called “fish arsenic”) that is much less harmful.
Arsenic that is attached to very small particles may stay in the air for many days and travel long distances. Many common arsenic compounds can dissolve in water. Thus, arsenic can get into lakes, rivers, or underground water by dissolving in rain or snow or through the discharge of industrial wastes. Some of the arsenic will stick to particles in the water or sediment on the bottom of lakes or rivers, and some will be carried along by the water. Ultimately, most arsenic ends up in the soil or sediment. Although some fish and shellfish take in arsenic, which may build up in tissues, most of this arsenic is in an organic form called arsenobetaine (commonly called “fish arsenic”) that is much less harmful.
 Newly CCA-treated wood may have some pesticide residue left on the wood surface from the treatment process. Because CCA is water-soluble, rainwater can seep in and leach CCA onto the wood surface. The CCA residue can be wiped or dislodged from the wood surface and can stick to hands or clothing from contact with the wood surface. Furthermore, the soil beneath and adjacent to CCA-treated wood structures has been shown to be contaminated by arsenic, chromium, and copper. When decks built with CCA-treated wood was coated with a waterproof sealant, the soil underneath had lower concentrations of the metal. Top of Page
Newly CCA-treated wood may have some pesticide residue left on the wood surface from the treatment process. Because CCA is water-soluble, rainwater can seep in and leach CCA onto the wood surface. The CCA residue can be wiped or dislodged from the wood surface and can stick to hands or clothing from contact with the wood surface. Furthermore, the soil beneath and adjacent to CCA-treated wood structures has been shown to be contaminated by arsenic, chromium, and copper. When decks built with CCA-treated wood was coated with a waterproof sealant, the soil underneath had lower concentrations of the metal. Top of Page
Exposure to Arsenic
Since arsenic is found naturally in the environment, you will be exposed to some arsenic by eating food, drinking water, or breathing air.
Children may also be exposed to arsenic by eating soil. Analytical methods used by scientists to determine the levels of arsenic in the environment generally do not determine the specific form of arsenic present. Therefore, we do not always know the form of arsenic a person may be exposed to. Similarly, we often do not know what forms of arsenic are present at hazardous waste sites. Some forms of arsenic may be so tightly attached to particles or embedded in minerals that they are not taken up by plants and animals.


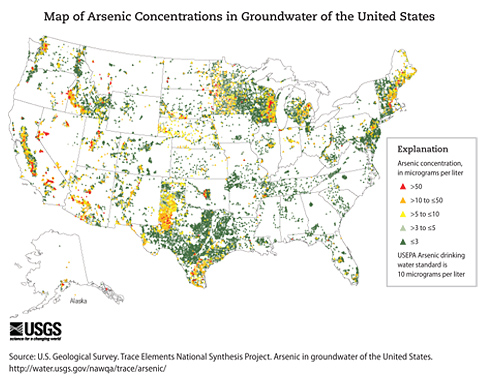
The concentration of arsenic in soil varies widely, generally ranging from about 1 to 40 parts of arsenic to a million parts of soil (ppm) with an average level of 3–4 ppm. However, soils in the vicinity of arsenic-rich geological deposits, some mining and smelting sites, or agricultural areas where arsenic pesticides had been applied in the past may contain much higher levels of arsenic. The concentration of arsenic in natural surface and groundwater is generally about 1 part in a billion parts of water (1 ppb), but may exceed 1,000 ppb in contaminated areas or where arsenic levels in soil are high. Groundwater is far more likely to contain high levels of arsenic than surface water. Surveys of U.S. drinking water indicate that about 80% of water supplies have less than 2 ppb of arsenic, but 2% of supplies exceed 20 ppb of arsenic. Levels of arsenic in food range from about 20 to 140 ppb. However, levels of inorganic arsenic, the form of most concern, are far lower. Levels of arsenic in the air generally range from less than 1 to about 2,000 nanograms (1 nanogram equals a billionth of a gram) of arsenic per cubic meter of air (less than 1–2,000 ng/m3), depending on location, weather conditions, and the level of industrial activity in the area. However, urban areas generally have mean arsenic levels in air ranging from 20 to 30 ng/m3.
You normally take in small amounts of arsenic in the air you breathe, the water you drink, and the food you eat. Of these, food is usually the largest source of arsenic. The predominant dietary source of arsenic is seafood, followed by rice/rice cereal, mushrooms, and poultry. Some seaweeds may contain arsenic in inorganic forms that may be more harmful. The total amount of arsenic you take in from these sources is generally about 50 micrograms (1 microgram equals one-millionth of a gram) each day. The level of inorganic arsenic (the form of most concern) you take in from these sources is generally about 3.5 microgram/day. Children may be exposed to small amounts of arsenic from handto- mouth activities from playing on play structures or decks constructed out of CCA-treated wood; however, this exposure is generally smaller than that they would receive from food and water. Hand washing can reduce the potential exposure of children to arsenic after playing on play structures constructed with CCA-treated wood, since most of the arsenic on the children’s hands is removed with water. Top of Page
Arsenic and the Body
 If you swallow arsenic in water, soil, or food, most of the arsenic may quickly enter into your body.
If you swallow arsenic in water, soil, or food, most of the arsenic may quickly enter into your body.
The amount that enters your body will depend on how much you swallow and the kind of arsenic that you swallow. This is the most likely way for you to be exposed near a waste site. If you breathe air that contains arsenic dusts, many of the dust particles settle onto the lining of the lungs. Most of the arsenic in these particles is then taken up from the lungs into the body. If you get arsenic-contaminated soil or water on your skin, only a small amount will go through your skin into your body, so this is usually not of concern.
Both inorganic and organic forms leave your body in your urine. Most of the inorganic arsenic will be gone within several days, although some will remain in your body for several months or even longer. If you are exposed to organic arsenic, most of it will leave your body within several days.
Health Effects of Exposure
Inorganic arsenic has been recognized as a human poison since ancient times.
Large oral doses (above 60,000 ppb in water which is 10,000 times higher than 80% of U.S. drinking water arsenic levels) can result in death. If you swallow lower levels of inorganic arsenic (ranging from about 300 to 30,000 ppb in water; 100–10,000 times higher than most U.S. drinking water levels), you may experience irritation of your stomach and intestines, with symptoms such as stomachache, nausea, vomiting, and diarrhea. Other effects you might experience from swallowing inorganic arsenic include decreased production of red and white blood cells, which may cause fatigue, abnormal heart rhythm, blood-vessel damage resulting in bruising, and impaired nerve function causing a “pins and needles” sensation in your hands and feet.
Perhaps the single-most characteristic effect of long-term oral exposure to inorganic arsenic is a pattern of skin changes. These include patches of darkened skin and the appearance of small “corns” or “warts” on the palms, soles, and torso, and are often associated with changes in the blood vessels of the skin. Skin cancer may also develop. Swallowing arsenic has also been reported to increase the risk of cancer in the liver, bladder, and lungs.

If you breathe high levels of inorganic arsenic, then you are likely to experience a sore throat and irritated lungs. You may also develop some of the skin effects mentioned above. The exposure level that produces these effects is uncertain, but it is probably above 100 micrograms of arsenic per cubic meter (μg/m3) for a brief exposure. Longer exposure at lower concentrations can also to circulatory and peripheral nervous disorders. There are some data suggesting that inhalation of inorganic arsenic may also interfere with normal fetal development, although this is not certain. An important concern is the ability of inhaled inorganic arsenic to increase the risk of lung cancer. This has been seen mostly in workers exposed to arsenic at smelters, mines, and chemical factories, but also in residents living near smelters and arsenical chemical factories. People who live near waste sites with arsenic may have an increased risk of lung cancer as well.
If you have direct skin contact with high concentrations of inorganic arsenic compounds, your skin may become irritated, with some redness and swelling. However, it does not appear that skin contact is likely to lead to any serious internal effects.
Almost no information is available on the effects of organic arsenic compounds in humans. Studies in animals show that most simple organic arsenic compounds (such as methyl and dimethyl compounds) are less toxic than the inorganic forms. In animals, ingestion of methyl compounds can result in diarrhea, and lifetime exposure can damage the kidneys. Lifetime exposure to dimethyl compounds can damage the urinary bladder and the kidneys.
Arsenic and Children
Children are exposed to arsenic in many of the same ways that adults are.
 Since children tend to eat or drink less of a variety of foods and beverages than do adults, ingestion of contaminated food or juice or infant formula made with arseniccontaminated water may represent a significant source of exposure. In addition, since children often play in the soil and put their hands in their mouths and sometimes intentionally eat soil, ingestion of contaminated soil may be a more important source of arsenic exposure for children than for adults. In areas of the United States where natural levels of arsenic in the soil and water are high, or in areas in and around contaminated waste sites, exposure of children to arsenic through ingestion of soil and water may be significant. In addition, contact with adults who are wearing clothes contaminated with arsenic (e.g., with dust from copper- or lead-smelting factories, from wood-treating or pesticide application, or from arsenic-treated wood) could be a source of exposure. Because of the tendency of children to taste things that they find, accidental poisoning from ingestion of pesticides is also a possibility. Thus, although most of the exposure pathways for children are the same as those for adults, children may be at a higher risk of exposure because of normal hand-tomouth activity.
Since children tend to eat or drink less of a variety of foods and beverages than do adults, ingestion of contaminated food or juice or infant formula made with arseniccontaminated water may represent a significant source of exposure. In addition, since children often play in the soil and put their hands in their mouths and sometimes intentionally eat soil, ingestion of contaminated soil may be a more important source of arsenic exposure for children than for adults. In areas of the United States where natural levels of arsenic in the soil and water are high, or in areas in and around contaminated waste sites, exposure of children to arsenic through ingestion of soil and water may be significant. In addition, contact with adults who are wearing clothes contaminated with arsenic (e.g., with dust from copper- or lead-smelting factories, from wood-treating or pesticide application, or from arsenic-treated wood) could be a source of exposure. Because of the tendency of children to taste things that they find, accidental poisoning from ingestion of pesticides is also a possibility. Thus, although most of the exposure pathways for children are the same as those for adults, children may be at a higher risk of exposure because of normal hand-tomouth activity.
The Children who are exposed to inorganic arsenic may have many of the same effects as adults, including irritation of the stomach and intestines, blood vessel damage, skin changes, and reduced nerve function. Thus, all health effects observed in adults are of potential concern in children. There is also some evidence that suggests that long-term exposure to inorganic arsenic in children may result in lower IQ scores. We do not know if absorption of inorganic arsenic from the gut in children differs from adults. There is some evidence that exposure to arsenic in early life (including gestation and early childhood) may increase mortality in young adults.
Young children are more at risk of exposure to CCA because they tend to spend more time playing outdoors, and because they have frequent hand-to-mouth activities. When playing on playground equipment or decks built with CCA-treated wood, they can be exposed to CCA by touching the CCA leachate on the wood surface with their hands and then inadvertently ingesting the CCA on their hands by hand-to-mouth activity.

There is some evidence that inhaled or ingested inorganic arsenic can injure pregnant women or their unborn babies, although the studies are not definitive. Studies in animals show that large doses of inorganic arsenic that cause illness in pregnant females can also cause low birth weight, fetal malformations, and even fetal death. Arsenic can cross the placenta and has been found in fetal tissues. Arsenic is found at low levels in breast milk. The dose levels that cause these effects also result in effects in the mothers. Top of Page
Reducing Risk of Exposure
If your doctor finds that you have been exposed to substantial amounts of arsenic, ask whether your children might also have been exposed. Your doctor might need to ask your state health department to investigate.
Many communities may have high levels of arsenic in their drinking water, particularly from private wells, because of contamination or as a result of the geology of the area. The north central region and the western region of the United States have the highest arsenic levels in surface water and groundwater sources, respectively. Wells used to provide water for drinking and cooking should be tested for arsenic. As of January 2006, EPA’s Maximum Contaminant Level (MCL) for arsenic in drinking water is 10 ppb. If you have arsenic in your drinking water at levels higher that the EPA’s MCL, an alternative source of water used for drinking and cooking should be considered.
 If you use arsenic-treated wood in home projects, personal protection from exposure to arsenic-containing sawdust may be helpful in limiting exposure of family members. These measures may include dust masks, gloves, and protective clothing. Arsenic-treated wood should never be burned in open fires, or in stoves, residential boilers, or fire places, and should not be composted or used as mulch. EPA’s Consumer Awareness Program (CAP) for CCA is a voluntary program established by the manufacturers of CCA products to inform consumers about the proper handling, use, and disposal of CCAtreated wood.
If you use arsenic-treated wood in home projects, personal protection from exposure to arsenic-containing sawdust may be helpful in limiting exposure of family members. These measures may include dust masks, gloves, and protective clothing. Arsenic-treated wood should never be burned in open fires, or in stoves, residential boilers, or fire places, and should not be composted or used as mulch. EPA’s Consumer Awareness Program (CAP) for CCA is a voluntary program established by the manufacturers of CCA products to inform consumers about the proper handling, use, and disposal of CCAtreated wood.
If you live in an area with a high level of arsenic in the water or soil, substituting cleaner sources of water and limiting contact with soil (for example, through use of a dense groundcover or thick lawn) would reduce family exposure to arsenic. By paying careful attention to dust and soil control in the home (air filters, frequent cleaning), you can reduce family exposure to contaminated soil. Make sure children wash their hands frequently and before eating. Discourage them from putting their hands in their mouths or engaging in other handto- mouth activities. Since arsenic may be found in the home as a pesticide, household chemicals containing arsenic should be stored out of reach of young children to prevent accidental poisonings. Always store household chemicals in their original labeled containers; never store household chemicals in containers that children would find attractive to eat or drink from, such as old soda bottles. Keep your Poison Control Center’s number by the phone.
It is sometimes possible to carry arsenic from work on your clothing, skin, hair, tools, or other objects removed from the workplace. This is particularly likely if you work in the fertilizer, pesticide, glass, or copper/lead smelting industries. You may contaminate your car, home, or other locations outside work where children might be exposed to arsenic. You should know about this possibility if you work with arsenic.
Material safety data sheets (MSDS) for many chemicals used should be found at your place of work, as required by the Occupational Safety and Health Administration (OSHA) in the U.S. Department of Labor. MSDS information should include chemical names and hazardous ingredients, and important properties, such as fire and explosion data, potential health effects, how you get the chemical(s) in your body, how to properly handle the materials, and what to do in the case of emergencies. Your state OSHA-approved occupational safety and health program or OSHA can answer any further questions and help your employer identify and correct problems with hazardous substances.
Exposure Testing
Several sensitive and specific tests can measure arsenic in your blood, urine, hair, or fingernails, and these tests are often helpful in determining if you have been exposed to aboveaverage levels of arsenic in the past.
These tests are not usually performed in a doctor’s office. They require sending the sample to a testing laboratory.
Measurement of arsenic in your urine is the most reliable means of detecting arsenic exposures that you experienced within the last several days. Most tests measure the total amount of arsenic present in your urine. This can sometimes be misleading, because the nonharmful forms of arsenic in fish and shellfish can give a high reading even if you have not been exposed to a toxic form of arsenic. For this reason, laboratories sometimes use a more complicated test to separate “fish arsenic” from other forms. Because most arsenic leaves your body within a few days, analysis of your urine cannot detect if you were exposed to arsenic in the past. Tests of your hair or fingernails can tell if you were exposed to high levels over the past 6–12 months, but these tests are not very useful in detecting low-level exposures. If high levels of arsenic are detected, this shows that you have been exposed, but unless more is known about when you were exposed and for how long, it is usually not possible to predict whether you will have any harmful health effects.
Governmental Recommendations
Recommendations and regulations are also updated periodically as more information becomes available.
For the most current information, check with the federal agency or organization that provides it for the substance in which you are interested. Some regulations and recommendations for arsenic include the following:
The federal government has taken several steps to protect humans from arsenic. First, EPA has set limits on the amount of arsenic that industrial sources can release into the environment. Second, EPA has restricted or canceled many of the uses of arsenic in pesticides and is considering further restrictions. Third, in January 2001, the EPA lowered the limit for arsenic in drinking water from 50 to 10 ppb. Finally, OSHA has established a permissible exposure limit (PEL), 8-hour time-weighted average, of 10 μg/m3 for airborne arsenic in various workplaces that use inorganic arsenic.
Additional Information
If you have any more questions or concerns, please contact your community or state health or environmental quality department or contact ATSDR at the address and phone number below.
Agency for Toxic Substances and Disease Registry
Division of Toxicology and Human Health Sciences
1600 Clifton Road NE, Mailstop F-57
Atlanta, GA 30329-4027
Toll-free information and technical assistance: 1-800-CDCINFO (1-800-232-4636)

- Page last reviewed: October 14, 2015
- Page last updated: October 14, 2015
- Content source:



 ShareCompartir
ShareCompartir