Vinyl chloride
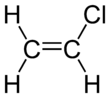
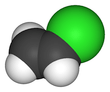
Vinyl chloride is an organochloride with the formula H2C=CHCl that is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC). About 13 billion kilograms are produced annually. VCM is among the top twenty largest petrochemicals (petroleum-derived chemicals) in world production.[2] The United States currently remains the largest VCM manufacturing region because of its low-production-cost position in chlorine and ethylene raw materials. China is also a large manufacturer and one of the largest consumers of VCM.[3] Vinyl chloride is a gas with a sweet odor. It is highly toxic, flammable, and carcinogenic. It can be formed in the environment when soil organisms break down chlorinated solvents. Vinyl chloride that is released by industries or formed by the breakdown of other chlorinated chemicals can enter the air and drinking water supplies. Vinyl chloride is a common contaminant found near landfills.[4] In the past VCM was used as a refrigerant.[5]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Chloroethene | |||
| Other names
Vinyl chloride monomer VCM Chloroethylene Refrigerant-1140 | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.756 | ||
| KEGG | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
C2H3Cl | ||
| Molar mass | 62.50 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | pleasant[1] | ||
| Density | 0.911 g/ml | ||
| Melting point | −153.8 °C (−244.8 °F; 119.3 K) | ||
| Boiling point | −13.4 °C (7.9 °F; 259.8 K) | ||
Solubility in water |
2.7 g/L (0.0432 mol/L) | ||
| Vapor pressure | 2580 mm. of mercury 20 °C (68 °F) | ||
Magnetic susceptibility (χ) |
-35.9·10−6 cm3/mol | ||
| Thermochemistry | |||
Heat capacity (C) |
0.8592 J/K/g (gas) 0.9504 J/K/g (solid) | ||
Std enthalpy of formation (ΔfH⦵298) |
−94.12 kJ/mol (solid) | ||
| Hazards | |||
EU classification (DSD) (outdated) |
|||
| R-phrases (outdated) | R12, R45 | ||
| S-phrases (outdated) | S45, S53 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | −61 °C (−78 °F; 212 K) | ||
| Explosive limits | 3.6%-33%[1] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 1 ppm C 5 ppm [15-minute][1] | ||
REL (Recommended) |
Ca[1] | ||
IDLH (Immediate danger) |
Ca [N.D.][1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Uses
Vinyl chloride is a chemical intermediate, not a final product. Due to the hazardous nature of vinyl chloride to human health there are no end products that use vinyl chloride in its monomer form. Polyvinyl chloride (PVC) is very stable, storable, and nowhere near as acutely toxic as the vinyl chloride monomer (VCM).
Vinyl chloride liquid is fed to polymerization reactors where it is converted from a monomeric VCM to a polymeric PVC. The final product of the polymerization process is PVC in either a flake or pellet form. From its flake or pellet form PVC is sold to companies that heat and mold the PVC into end products such as PVC pipe and bottles. Several million tons of PVC are sold on the global market each year.
Until 1974, vinyl chloride was used in aerosol spray propellant.[6] Vinyl chloride was briefly used as an inhalational anaesthetic, in a similar vein to ethyl chloride, though its toxicity forced this practice to be abandoned.
Smaller amounts of vinyl chloride are used in furniture and automobile upholstery, wall coverings, housewares, and automotive parts. Vinyl chloride has also been used in the past as a refrigerant.[7]
Production
Vinyl chloride was first produced in 1835 by Justus von Liebig and his student Henri Victor Regnault. They obtained it by treating 1,2-dichloroethane with a solution of potassium hydroxide in ethanol.[8]
In 1912, Fritz Klatte, a German chemist working for Griesheim-Elektron, patented a means to produce vinyl chloride from acetylene and hydrogen chloride using mercuric chloride as a catalyst. While this method was widely used during the 1930s and 1940s in the West, it has since been superseded by more economical processes based on ethylene in the United States and Europe. It remains the main production method in China.
Vinyl chloride is produced on a substantial scale—approximately 31.1 million tons were produced in 2000.[9] Two methods are employed, the hydrochlorination of acetylene and the dehydrochlorination of ethylene dichloride (1,2-dichloroethane). Numerous attempts have been made to convert ethane directly to vinyl chloride.[2]
Vinyl chloride can also be obtained as a byproduct in the synthesis of chlorofluorocarbons when saturated chlorofluorocarbons are catalytically dechlorinated by ethylene. Ethane sulfochlorination has been proposed as a route to produce vinyl chloride using sulfur instead of oxygen.[2]
Thermal decomposition of dichloroethane
1,2-Dichloroethane ("dichloroethane", (C2H4Cl2)) is cheaply produced from inexpensive ethane. When heated to 500 °C at 15–30 atm (1.5 to 3 MPa) pressure, dichloroethane decomposes to produce vinyl chloride and anhydrous HCl.
- ClCH2CH2Cl → CH2=CHCl + HCl
Due to the relatively low cost of dichloroethane compared to acetylene, most vinyl chloride has been produced via this technique since the late 1950s.
The thermal cracking reaction is highly endothermic, and is generally carried out in a fired heater. Even though residence time and temperature are carefully controlled, it produces significant quantities of chlorinated hydrocarbon side products. In practice, EDC conversion is relatively low (50 to 60 percent). The furnace effluent is immediately quenched with cold EDC to stop undesirable side reactions. The resulting vapor-liquid mixture then goes to a purification system. Some processes use an absorber-stripper system to separate HCl from the chlorinated hydrocarbons, while other processes use a refrigerated continuous distillation system.
Production from acetylene
Acetylene reacts with anhydrous hydrogen chloride gas over a mercuric chloride catalyst to give vinyl chloride:
- C2H2 + HCl → CH2=CHCl
The reaction is exothermic and highly selective. Product purity and yields are generally very high.
This industrial route to vinyl chloride was common before ethylene became widely distributed. When vinyl chloride producers shifted to using the thermal cracking of EDC described above, some used byproduct HCl in conjunction with a colocated acetylene-based unit. The hazards of storing and shipping acetylene meant that the vinyl chloride facility needed to be located very close to the acetylene generating facility. China still uses this method to produce vinyl chloride due to the large reserves of coal from which acetylene is produced.[3]
Production from ethane
Ethane is readily available, particularly on the U.S. gulf coast. Ethylene is made from ethane by cracking ethane and then ethylene is used for production of vinyl chloride. Hence, to save the processing cost for manufacturing ethylene, numerous attempts have been made to convert ethane directly to vinyl chloride. The direct feed of ethane to vinyl chloride plants could thus considerably decrease the raw material costs and make the plants less dependent on cracker capacity. The conversion of ethane to vinyl chloride can be performed by various routes:[10]
High-temperature chlorination:
- C2H6 + 2 Cl2 → C2H3Cl + 3 HCl
High-temperature oxychlorination:
- C2H6 + HCl + O2 → C2H3Cl + 2 H2O
High-temperature oxidative chlorination:
- 2 C2H6 + 3⁄2 O2 + Cl2 → 2 C2H3Cl + 3 H2O
A major drawback to the use of ethane are the forcing conditions required for its use, which can be attributed to its lack of molecular functionality. In contrast to ethylene, which easily undergoes chlorine addition, ethane must first be functionalized by substitution reactions, which gives rise to a variety of consecutive and side-chain reactions. The reaction must, therefore, be kinetically controlled in order to obtain a maximal vinyl chloride yield. Vinyl chloride yields average 20–50% per pass. Ethylene, ethyl chloride, and 1,2-dichloroethane are obtained as major byproducts. With special catalysts and at optimized conditions, however, ethane conversions of greater than 96% have been reported from oxychlorination reactions. The ethylene formed can either be recycled or oxychlorinated and cracked in a conventional manner. Many such ethane-based processes have been and are being developed.
Storage and transportation
Vinyl chloride is stored as a liquid. The presently accepted upper limit of safety as a health hazard is 500 ppm. Often, the storage containers for the product vinyl chloride are high capacity spheres. The spheres have an inside sphere and an outside sphere. Several inches of empty space separate the inside sphere from the outside sphere. This void area between the spheres is purged with an inert gas such as nitrogen. As the nitrogen purge gas exits the void space it passes through an analyzer that is designed to detect if any vinyl chloride is leaking from the internal sphere. If vinyl chloride starts to leak from the internal sphere or if a fire is detected on the outside of the sphere then the contents of the sphere are automatically dumped into an emergency underground storage container. Containers used for handling vinyl chloride at atmospheric temperature are always under pressure. Inhibited vinyl chloride may be stored at normal atmospheric conditions in suitable pressure vessel. Uninhibited vinyl chloride may be stored either under refrigeration or at normal atmospheric temperature in the absence of air or sunlight but only for a duration of a few days. If for longer periods, regular checks should be made for the presence of polymers.[11]
Transporting VCM presents the same risks as transporting other flammable gases such as propane, butane (LPG) or natural gas (for which the same safety regulations apply). The equipment used for VCM transport is specially designed to be impact and corrosion resistant.[12]
Fire and explosion hazard
In the U.S., OSHA lists vinyl chloride as a Class IA Flammable Liquid, with a National Fire Protection Association Flammability Rating of 4. Because of its low boiling point, liquid VCM will undergo flash evaporation (i.e., autorefrigerate) upon its release to atmospheric pressure. The portion vaporized will form a dense cloud (more than twice as heavy as the surrounding air). The risk of subsequent explosion or fire is significant. According to OSHA, the flash point of vinyl chloride is −78 °C (−108.4 °F).[13] Its flammable limits in air are: lower 3.6 volume% and upper 33.0 volume%. The explosive limits are: lower 4.0%, upper 22.05 by volume in air. Fire may release toxic hydrogen chloride (HCl) and carbon monoxide (CO).[14] VCM can polymerise rapidly due to heating and under the influence of air, light and contact with a catalyst, strong oxidisers and metals such as copper and aluminium, with fire or explosion hazard. As a gas mixed with air, VCM is a fire and explosion hazard. On standing VCM can form peroxides, which may then explode. VCM will react with iron and steel in the presence of moisture.[5][15] Vinyl chloride is a gas at normal atmospheric temperature and pressure.
It is flammable, emitting hydrogen chloride.
Health effects
Vinyl chloride finds its major application in the production of PVC. It is volatile, so the primary exposure is via inhalation as against food or water with occupational hazards being highest. Prior to 1974, workers were commonly exposed to 1,000 ppm vinyl chloride, causing "vinyl chloride illness" such as acroosteolysis and Raynaud's Phenomenon. The symptoms of vinyl chloride exposure are classified by ppm levels in ambient air with 4,000 ppm having a threshold effect.[16] The intensity of symptoms varies from acute (1,000-8,000 ppm), including dizziness, nausea, visual disturbances, headache, and ataxia, to chronic (above 12,000 ppm), including narcotic effect, cardiac arrhythmias, and fatal respiratory failure.[17] RADS (Reactive Airway Dysfunction Syndrome) may be caused by acute exposure to vinyl chloride.[18]
Vinyl chloride is a mutagen having clastogenic effects which affect lymphocyte chromosomal structure.[17][19] Vinyl chloride is a Group 1 human carcinogen posing elevated risks of rare angiosarcoma, brain and lung tumors, and malignant haematopoeitic lymphatic tumors.[20] Chronic exposure leads to common forms of respiratory failure (emphysema, pulmonary fibrosis) and focused hepatotoxicity (hepatomegaly, hepatic fibrosis). Continuous exposure can cause CNS depression including euphoria and disorientation. Decreased male libido, spontaneous abortion, and birth defects are known major reproductive defects associated with vinyl chloride.
Vinyl chloride can have acute dermal and ocular effects. Dermal exposure effects are thickening of skin, edema, decreased elasticity, local frostbites, blistering, and irritation.[17] The complete loss of skin elasticity expresses itself in Raynaud’s Phenomenon.[19]
The US OSHA limits vinyl chloride exposure of workers to no more than 1 ppm for eight hours or 5 ppm for 15 minutes. The US EPA and FDA limit vinyl chloride in drinking water to 0.002 ppm. Food (ingestion) is a trivial source of exposure.
Liver toxicity
The hepatotoxicity of vinyl chloride has long been established since the 1930s when the PVC industry was just in its infant stages. In the very first study about the dangers of vinyl chloride, published by Patty in 1930, it was disclosed that exposure of test animals to just a single short-term high dose of vinyl chloride caused liver damage.[21] In 1949, a Russian publication discussed the finding that vinyl chloride caused liver injury among workers.[22] In 1954, B.F. Goodrich Chemical stated that vinyl chloride caused liver injury upon short-term exposures. Almost nothing was known about its long-term effects. They also recommended long-term animal toxicology studies. The study noted that if a chemical did justify the cost of testing, and its ill-effects on workers and the public were known, the chemical should not be made.[23] In 1963, research paid for in part by Allied Chemical found liver damage in test animals from exposures below 500 parts per million (ppm).[24] Also in 1963, a Romanian researcher published findings of liver disease in vinyl chloride workers.[25] In 1968, Mutchler and Kramer, two Dow researchers, reported their finding that exposures as low as 300 ppm caused liver damage in vinyl chloride workers thus confirming earlier animal data in humans.[26] In a 1969 presentation given in Japan, P. L. Viola, a European researcher working for the European vinyl chloride industry, indicated, "every monomer used in V.C. manufacture is hazardous....various changes were found in bone and liver. Particularly, much more attention should be drawn to liver changes. The findings in rats at the concentration of 4 to 10 ppm are shown in pictures." In light of the finding of liver damage in rats from just 4–10 ppm of vinyl chloride exposure, Viola added that he "should like some precautions to be taken in the manufacturing plants polymerizing vinyl chloride, such as a reduction of the threshold limit value of monomer …"[27]
Cancerous tumors
In 1970, Viola reported that test animals exposed to 30,000 ppm of vinyl chloride developed cancerous tumors. Viola began his research looking for the cause of liver and bone injuries found in vinyl chloride workers. Viola's findings in 1970 were a "red flag" to B.F. Goodrich and the industry.[28] In 1972, Maltoni, another Italian researcher for the European vinyl chloride industry, found liver tumors (including angiosarcoma) from vinyl chloride exposures as low as 250 ppm for four hours a day.[29]
In the late 1960s, the cancers that all of these studies warned of finally manifested themselves in workers. John Creech from B.F. Goodrich discovered angiosarcoma (a very rare cancer) in the liver of a worker at the B.F. Goodrich plant in Louisville, Kentucky. Then, finally, on January 23, 1974, B.F. Goodrich informed the government and issued a press release stating that it was "investigating whether the cancer deaths of three employees in the polyvinyl chloride operations at its Louisville, Ky. plant were related to occupational causes."
In 1997 the U.S. Centers for Disease Control and Prevention (CDC) concluded that the development and acceptance by the PVC industry of a closed loop polymerization process in the late 1970s "almost completely eliminated worker exposures" and that "new cases of hepatic angiosarcoma in vinyl chloride polymerization workers have been virtually eliminated."[30]
The Houston Chronicle claimed in 1998 that the vinyl industry manipulated vinyl chloride studies to avoid liability for worker exposure and hid extensive and severe chemical spills in local communities.[31]
Environment pollution
According to the United States Environmental Protection Agency (EPA), "vinyl chloride emissions from polyvinyl chloride (PVC), ethylene dichloride (EDC), and vinyl chloride monomer (VCM) plants cause or contribute to air pollution that may reasonably be anticipated to result in an increase in mortality or an increase in serious irreversible, or incapacitating reversible illness. Vinyl chloride is a known human carcinogen that causes a rare cancer of the liver."[32] EPA's 2001 updated Toxicological Profile and Summary Health Assessment for VCM in its Integrated Risk Information System (IRIS) database lowers EPA's previous risk factor estimate by a factor of 20 and concludes that "because of the consistent evidence for liver cancer in all the studies...and the weaker association for other sites, it is concluded that the liver is the most sensitive site, and protection against liver cancer will protect against possible cancer induction in other tissues."[33]
Additional references
- International Programme on Chemical Safety (IPCS) (1997). '"Vinyl chloride. Poisons Information Monograph. PIM 558. WHO. Geneva.
- National Poisons Information Service (NPIS) (2004). "Vinyl chloride." TOXBASE®.
- World Health Organisation (WHO) (2000). "Air quality guidelines for Europe." WHO Regional Publications, European Series, No. 91. 2nd edition. WHO Regional Office for Europe. Copenhagen.
- Hathaway G.J. and Proctor N.H. (2004). Chemical Hazards of the Workplace. 5th edition. John Wiley & Sons, New Jersey.
- Risk Assessment Information System (RAIS) (1993). "Toxicity summary for vinyl chloride. "Chemical Hazard Evaluation and Communication Group, Biomedical and Environmental Information Analysis Section, Health and Safety Research Division.
Microbial remediation
The bacteria species Nitrosomonas europaea can degrade a variety of halogenated compounds including trichloroethylene, and vinyl chloride.[34]
See also
- Vinyl group
References
- NIOSH Pocket Guide to Chemical Hazards. "#0658". National Institute for Occupational Safety and Health (NIOSH).
- E.-L. Dreher, T. R. Torkelson, K. K. Beutel "Chlorethanes and Chloroethylenes" in Ullmann's Encyclopedia of Industrial Chemistry 2011, Wiley-VCH, Weinheim. doi:10.1002/14356007.o06_o01
- "Vinyl Chloride Monomer (VCM) - Chemical Economics Handbook (CEH) - IHS Markit". www.ihs.com. Retrieved 5 April 2018.
- "http://www.dhs.wisconsin.gov/eh/chemfs/fs/vc.htm"
- "http://www.npi.gov.au/resource/vinyl-chloride-monomer-vcm"
- Markowitz, Gerald; Rosner, David (2013). Deceit and Denial: The Deadly Politics of Industrial Pollution. Berkeley, California Press: University of California Press. p. 185 – via Questia.
- http://www.epa.gov/ttnatw01/hlthef/vinylchl.html
- Regnault, H.V. (1835) Sur la Composition de la Liqueur des Hollandais et sur une nouvelle Substance éthérée. Annales de Chimie et de Physique, Gay-Lussac & Arago, Vol. 58, Paris, Crochard Libraire, 301–320 https://gallica.bnf.fr/ark:/12148/bpt6k6569005x/f307.item.texteImage
- Klaus Weissermel, Hans-Jürgen Arpe in "Industrial organic chemistry"
- Ullmann's Encyclopedia of Industrial Chemistry (Wiley, 2007)(ISBN 3527316027)(O)(28029s)_ChGe_-Chlorinated hydrocarbons
- "http://aseh.net/resources/restored/resources/teaching-units/teaching-unit-better-living-through-chemistry/historical-sources/lesson-1/MCA-Vinal%20Chloride%20Safety%20Sheet-1954.pdf"
- "http://www.pvc.org/en/p/vinyl-chloride-monomer-vcm"
- http://aseh.net/resources/restored/resources/teaching-units/teaching-unit-better-living-through-chemistry/historical-sources/lesson-1/MCA-Vinal%20Chloride%20Safety%20Sheet-1954.pdf
- "Occupational Safety and Health Guideline for Vinyl Chloride"1988."
- "Vinyl chloride: health effects, incident management and toxicology - GOV.UK". www.gov.uk. Retrieved 5 April 2018.
- Harrison, Henrietta (2008). HPA Report Version 1. CHAP DHQ
- International Programme on Chemical Safety (IPCS) (1999). Vinyl chloride. Environmental Health Criteria 215. WHO. Geneva.
- UK Department for Environment, Food, and Rural Affairs (DEFRA) and Environment Agency (EA) (2004). "Contaminants in soil: Collation of toxicological data and intake values for humans. Vinyl chloride."
- Agency for Toxic Substances and Disease Registry (ATSDR) (2006). "Toxicological Profile for vinyl chloride". US Department of Health and Human Services. Atlanta, US.
- International Agency for Research on Cancer (IARC). "Vinyl chloride, polyvinyl chloride, and vinyl chloride-vinyl acetate copolymers." Vol 19, 1979. IARC. "Vinyl chloride." Supplement 7, 1987. Lyon.
- Patty, F.A. et al. "Acute Response of Guinea Pigs to Vapors of Some Commercial Organic Compounds." Public Health Reports. Volume 45, Number 24. August 22, 1930
- Tribukh, S L et al. "Working Conditions and Measures for Their Improvement in Production and Use of Vinylchloride Plastics" (1949)
- Wilson, Rex H et al. "Toxicology of Plastics and Rubber- Plastomers and Monomers." Reprinted from Industrial Medicine and Surgery. 23:11, 479–786. November 1954.
- Lester, D. et al. "Effects of Single and Repeated Exposures of Humans and Rats to Vinyl Chloride" Laboratory of applied biodynamics, Yale University and Department of Pathology. School of Medicine, Yale University, New Haven, Connecticut.
- Suciu, I et al. "Clinical Manifestations in Vinyl Chloride Poisoning" Clinic of Professional Diseases. Cluj, Romania.
- Kramer, G.C., M.D. "The Correlation of Clinical and Environmental Measurements for Workers Exposed to Vinyl Chloride." The Dow Chemical Company. Midland Michigan.
- Viola, P.L. "Pathology of Vinyl Chloride" International Congress on Occupational Health. Japan. 1969.
- Viola, P L. "Carcinogenic Effect of Vinyl Chloride" Presented at the Tenth International Cancer Congress. Houston, Texas. May 22–29, 1970.
- Maltoni, C. "Cancer Detection and Prevention" (1972) Presented at the Second International Symposium on Cancer Detection and Prevention. Bologna, April 9–12, 1973.
- Epidemiologic Notes and Reports Angiosarcoma of the Liver Among Polyvinyl Chloride Workers – Kentucky. Centers for Disease Control and Prevention. 1997.
- Jim Morris, "In Strictest Confidence. The chemical industry's secrets," Houston Chronicle. Part One: "Toxic Secrecy," June 28, 1998, pgs. 1A, 24A–27A; Part Two: "High-Level Crime," June 29, 1998, pgs. 1A, 8A, 9A; and Part Three: "Bane on the Bayou," July 26, 1998, pgs. 1A, 16A.
- National Emission Standards for Hazardous Air Pollutants (NESHAP) for Vinyl Chloride Subpart F, OMB Control Number 2060-0071, EPA ICR Number 0186.09 (Federal Register: September 25, 2001 (Volume 66, Number 186))
- EPA Toxicological Review of Vinyl Chloride in Support of Information on the IRIS. May 2000
- "Home - Nitrosomonas europaea". genome.jgi-psf.org. Retrieved 5 April 2018.
Further reading
- "Medicine: The Plastic Peril". Time. May 13, 1974. Retrieved 2 July 2010.