Universal neonatal hearing screening
Universal neonatal hearing screening (UNHS), also known as early hearing detection and intervention (EHDI) programs in several countries, refer to those services aimed at the early identification, intervention, and follow-up of infants and young children who are deaf or hard-of-hearing. It is a strategy for early detection of permanent congenital hearing loss. It describes the use of objective testing methods (usually otoacoustic emission (OAE) testing or automated auditory brainstem response (ABR) testing) to screen the hearing of well newborns in a particular target region.
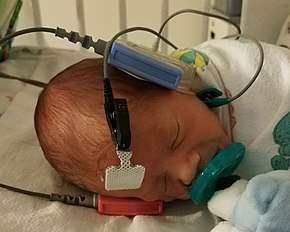
Overview
Even among developed countries, until the 90's, it could take years for hearing-impaired child to be diagnosed and to benefit from a health intervention and amplification. This delay still can happen in developing countries.[1] If children are not exposed to sounds and language during their first years of life because of a hearing loss, they will have difficulty in developing spoken or signed language; cognitive development and social skills could also be affected.[2]
This screening separates children into two groups—those with a high index of suspicion (more likely to have permanent congenital hearing loss) and those with a low index of suspicion (less likely to have permanent congenital hearing loss). Those in the first group are referred for diagnostic testing.[3]
Newborn hearing screening is becoming popular as it aims to reduce the age of detection for hearing loss—meaning that diagnosed children can receive early intervention, which is more effective because the brain's ability to learn language (spoken, cued, or signed) reduces as the child ages.[4] Children born with permanent congenital hearing loss have historically performed worse educationally, had poorer language acquisition, social functioning and vocational choices than their hearing peers.[5][6][7][8]
In order to be most effective in minimizing developmental delays and promoting communication, education and social development, timely and appropriate interventions need to follow the early identification of hearing loss. Interventions for children with permanent congenital hearing loss ranges from devices that amplify sound to devices that replace the function of a damaged inner ear to communication modalities including spoken language, sign language, and Cued Speech. The choice of interventions depends on the degree and the cause of hearing loss, accessibility, affordability and family choice. For interventions to be effective, they should be appropriate, timely, family-centered and undertaken through a coordinated interdisciplinary approach, which includes access to specialists who have the professional qualifications and specialized knowledge and skills to support and promote optimal development outcomes.[9] Key elements for ensuring the best outcomes for children with hearing loss may include:[10]
- hearing devices, such as hearing aids, and middle ear or cochlear implants;
- hearing assistive technology, such as FM/radio systems and loop systems;
- therapy to develop spoken language, such as auditory-verbal therapy, cued speech and auditory-oral therapy;
- development of nonverbal communication, such as sign language (see also American Sign Language).
Newborn hearing screening programmes exist in many countries, including the United States,[11] United Kingdom[12] New Zealand,[13] and the majority of countries making up the European Union.[14] In order to maximize language and communication competence, literacy development, and psychosocial well-being, the U.S. Joint Committee on Infant Hearing[15] endorses the goals that 1) all newborns should undergo hearing screening using physiologic measures prior to hospital discharge, but no later than one month of age 2) all infants whose do not pass screening should have appropriate audiologic diagnosis no later than 3 months of age and 3) all infants identified as deaf or hard of hearing in one or both ears should be referred to early targeted and appropriate intervention services as soon as possible after diagnosis, but no later than six months of age. Newborn hearing screening employs objective assessment methods, either with automated (ABR) or (OAE), or both for initial and/or rescreening procedures.[16]
Rates of congenital hearing loss
Hearing loss in neonates is the most common congenital sensory disorder, and can be caused by a variety of reasons. Research has placed the prevalence of significant permanent hearing loss in neonates at 1–2 per 1000 live births in the United States.[17][18] With this screening, many forms of congenital hearing loss can be detected. Congenital hearing loss can be due to genetic causes, environmental exposures during pregnancy, or health complications shortly after birth.[19] Population-based studies in Europe and North America have identified a consistent prevalence of approximately 0.1% of children having a hearing loss of more than 40 decibels through review of health or education records, or both. Other international studies using different methods or criteria have reported higher estimates.[20] In the United States, studies have shown a wide range of estimates for the number of children with hearing loss depending upon the reported age range, type, degree, frequency, laterality and method of ascertainment (e.g. audiometric testing, parental report, record review). Audiometric data of adolescents aged 12 to 19 years obtained through the National Health and Nutrition Examination Survey (NHANES) identified 3% to 5% of adolescents with hearing loss of 25 decibels or more and 15% to 20% with hearing losses of greater than 15 decibels.[21]
Research studies
Studies have found that early diagnosis and intervention for children with hearing loss can help them develop better communication skills. Researchers have shown children with hearing loss meeting the current early identification and intervention guidelines were more likely to have enhanced vocabulary than children who did not meet EHDI 1-3-6 guidelines.[22] Funded research projects have focused on improving EHDI process issues including improving LFU/LTD rates through at Women, Infants, and Children (WIC) certification screening visits.[23][24] and exploring technologies that can be incorporated in electrophysiological testing to facilitate hearing loss diagnosis in newborns, without the use of sedation.[25]
Investigators have reported on numerous topics including the screening and diagnostic evaluations of children with unilateral and mild bilateral hearing loss,[26] the impact of hearing loss and comorbidities,[27] and the long-term hearing loss risk of children born with cytomegalovirus.[28]
Screening methodology
Often a two-stage process occurs in the actual screening of the hearing. Children are screened with either otoacoustic emissions (OAE) or automated auditory brainstem response (AABR). Children passing the test receive no further assessment. Children who fail the initial screen are usually referred for a second screening assessment either with OAE's or AABR. Children failing this second assessment will usually be sent for diagnostic assessment of their hearing. There is some variation in procedure by region and country but most follow this basic principle.[1]
Screening personnel vary also, in some regions Audiologists are used, whereas technicians, nurses, or volunteers are used in other programs. In countries that have insufficient financial and human resources to implement hearing screening, community-based programs have used simple, behaviour-based questionnaires to identify infants with hearing loss but with limited success.[1]
Targeted hearing screening
Targeted neonatal hearing screening describes the process by which only a specific subset of a population are screened (for instance those infants in the neonatal intensive care unit or with risk factors for hearing loss). Although the U.S. Joint Committee on Infant Hearing (JCIH) endorsed the goal of universal detection of infants with hearing loss in 1994, it modified and maintained a role for specific high risk factors described in their previous (1973, 1982 and 1990) position statements.[29]
Successes
Universal newborn hearing screening programs aim to have high coverage rates (participation) and many aim to screen babies by 1 month of age, aim to complete the diagnostic process for referred babies by three months of age, and aim to begin intervention services by six months of age.[30]
U.S. jurisdictional programs (states and territories) screened less than 3% of all newborns for hearing loss in the U.S. at the beginning of the 1990s.[31] Data from states and territories for the early years of EHDI (1999 – 2004) were collected using surveys conducted by the Directors of Speech and Hearing Programs in State Health and Welfare Agencies[32] and shared with the CDC EHDI Program.[33][34] Beginning in 2005, CDC obtained data through an Office of Management and Budget approved survey sent to EHDI program directors. In 1999, 22 jurisdictions estimated that less than half of all infants (46.5%) were screened for hearing loss, steadily increasing to 80.1% in 2005 85.4% in 2007, and 98.0% by 2009.[35] The number of deaf and hard of hearing babies identified early in life in the U.S. has steadily increased from 855 in the year 2000, 2,634 in 2005 and most recently reported 6,337 in calendar year 2016.[36]
Challenges
In addition to meeting the “1-3-6” targets, one of the key challenges for newborn hearing screening programmes is to reduce 'loss to follow-up' (where a child doesn't return for the next stage of the process). The Joint Committee on Infant Hearing (US) has reported that this is a significant problem in state screening programmes in the United States and other jurisdictions. Measuring loss to follow-up is an important step in understanding and reducing it.[37]
Now that over 95% of U.S. infants are having their hearing screened, remaining challenges include ensuring timely diagnostic evaluation for those who do not pass the screening and enrollment in early intervention for those with diagnosed hearing loss. In 2005, >60% of infants who had not passed the final or most recent screening were lost-to-follow-up/lost to documentation (LFU/LTD).[38] Some of those infants may have received audiologic evaluations, but the results not reported to the EHDI program (i.e., undocumented evaluation). By 2007, LFU/LTD among infants not passing the final or most recent screening had decreased to approximately 46% and to 35% in 2011. The LFU/LTD percent for diagnosis in 2016 was 25.4% (n = 16,522). The 2016 LFU/LTD percent for enrollment in early intervention was 19.6% (n = 1,239).[35]
Challenges to newborn hearing screening have existed for over three decades.[39] Newborn screening alone can miss postnatal, progressive or acquired hearing loss, there is poor identification of perinatal infections, and concerns over regulatory barriers and privacy continue to this day.[40] Many infants are lost to follow-up and many families face the challenge of navigating coordinated quality care through complex health to education systems involving multiple agencies.[41][42][43]
History
In 1956, Erik Wedenberg published one of the earliest articles describing examiner use of tuning forks, percussion sounds, pitch pipes, and cowbells to screen the hearing newborn infants. The author noted, "until recently it has not been considered possible to carry out reliable auditory tests until the child has attained the age of 6–7 years."[44]
In 1963, Marion Downs, affectionately referred to as the "mother of pediatric audiology", pioneered the first hospital based infant hearing screening program in Denver, Colorado using Behavioral Observation Audiometry (BOA). Several independent observers recorded eye-blink and/or startle responses after presentation of narrow-band (90 dB) and white noise (93 dB) stimulation. In her 1964 publication, the observers identified suspected hearing losses, although disagreements were significant for 26% of the infants.[39] In 1969, Marion led efforts for the formation of the Joint Committee on Infant Hearing (JCIH) to provide multi-disciplinary leadership and guidance in all areas of newborn and infant hearing issues.[15]
In their 1971 Position Statement, the JCIH determined the results of screening programs were inconsistent and misleading and, although recognizing the urgent need for early detection, recommended discontinuing routine behavioral hearing screening of newborn infants. In 1973 the Committee recommended that only infants with certain high risk factors have their hearing evaluated (five factors: family history; congenital perinatal infections; ear, nose or throat defects; low birthweight <1500 g; hyperbilirubinemia).[45]
In 1982, two additional risk factors were added (bacterial meningitis and birth asphyxia including low Apgar scores) and included the recommendation for BOA or physiologic screening of high-risk infants. At that time, the Committee did not recommend any specific device, although many program were successfully utilizing automated ABR for newborn screening. Despite efforts and endorsements, the growth of high-risk screening in the United States was very slow.[46] In 1984, high-risk hearing registries only included an estimated 15 percent of the nation's newborn population and likely, that less than half of those infants had their hearing assessed. Other weaknesses identified that a restricted risk register will excludes approximately 50 percent of infants with hearing impairment.[47]
In 1989 Surgeon General C. Everett Koop, perhaps most remembered for his work related to abortion, tobacco, and AIDS, called for increased efforts to identify congenital hearing loss within the first year of life. In his words “It's a tall order, yes, but if we all work together, I believe we can fill it."[48]
In 1990, the JCIH added more 3 risk factors (ototoxic medication, prolonged mechanical ventilation, syndromic stigmata) for a total of ten. Congressman James T. Walsh (R-NY) sponsored and introduced the first attempt at federal legislation with the Hearing Loss Testing Act of 1991[49] requiring the hearing testing of every child born in the United States at the time of birth and establishing uniform standards for such testing. Although the Committee on Energy and Commerce referred this legislation to subcommittee, Congressman Walsh continued to promote legislation throughout that decade as the co-founder and co-chair of the Congressional Hearing Health Caucus. In 1993, the National Institutes of Health Consensus statement initiated the concept of “1-3-6” as the monthly milestones for screening, diagnosis and intervention. Between 1993 and 1996, the National Center for Hearing Assessment and Management at Utah State University conducted the Rhode Island Hearing Assessment Project (RIHAP), which demonstrated the feasibility of using transient OAEs for universal screening.[50][51] In 1994, both the JCIH and the DSHPSHWA endorsed universal newborn hearing screening. In 1996, the US Preventive Services Task Force concluded that the evidence was insufficient to assess the balance of benefits and harms and assigned an “I Statement” grade for newborn hearing screening.[52]
All Us States participate in Early Hearing Detection and Intervention (EHDI) Programs.[53] The “birth of EHDI” may be attributed to passage of the Children's Health Act of 2000 which included language from Walsh's proposed legislation and authorized the Secretary of the Department of Health and Human Services (HHS), acting through the Centers for Disease Control and Prevention and the Health Resources and Services AdministrationA, to establish “statewide newborn and infant hearing screening evaluation and intervention programs and systems.” Congress provided HRSA with the authority to support statewide services and CDC with the authority to provide technical assistance for data management and applied research. In addition, the National Institute on Deafness and Other Communication Disorders at NIH provided authority to continue a program of research and development on the efficacy of new screening techniques and technology.[54]
The year 2000 Position Statement of the JCIH provided the Principles and Guidelines for Early Hearing Detection and Intervention Programs. In 2006, the HHS Secretary's Advisory Committee on Heritable Disorders in Newborns and Children (SACHDNC) included newborn hearing as one of the conditions to be included in their Recommended Uniform Screening Panel (RUSP).[31] With growing research evidence, in 2007 United States Preventive Services Task Force (USPSTF) recommended screening of hearing loss in all newborn infants with an assigned “B grade.” By 2010, 43 states enacted legislative statutes or written regulatory language related to universal newborn hearing screening.[55]
The “electronic age of EHDI” may have begun during the next decade. The Quality, Research, and Public Health (QRPH) Planning and Technical Committees of Integrating the Healthcare Enterprise published a series of EHDI technical documents.[56] These profiles promote the automated collection and communication exchange of EHDI data between clinical and public health information systems (results, demographics, care plans, quality measures). The U.S. National Library of Medicine maintains the Newborn Screening Coding and Terminology Guide[57] to promote and facilitate the use of electronic health data standards in recording and transmitting newborn screening test results. This includes EHDI standard vocabulary codes and terminologies, including Logical Observation Identifiers Names and Codes (LOINC®), Systematized Nomenclature of Medicine — Clinical Terms (SNOMED CT®). The National Quality Forum the Centers for Medicare & Medicaid Services, and the Joint Commission for hospital accreditation have endorsed and/or supported adoption of EHDI electronic quality measures.[58] EHDI-PALS (Pediatric Audiology Links to Services) provides web-based link to information, resources, and services for children with hearing loss.[59] In 2018, Health Level 7 (HL7) approved the Early Hearing Detection and Intervention (EHDI) Implementation Guide as a Normative Standard.[60]
References
- McPherson B (2012). "Newborn hearing screening in developing countries: needs & new directions". The Indian Journal of Medical Research. 135: 152–3. PMC 3336843. PMID 22446854.
- "NIH Fact Sheets: Newborn Hearing Screening". U.S. National Institutes of Health. Retrieved 2019-03-01.
- Wilson JMG; Jungner G. "Principles and practice of screening for disease". World Health Organization. Retrieved 6 March 2019.
- Downs MP, Yoshinaga-Itano C (February 1999). "The efficacy of early identification and intervention for children with hearing impairment". Pediatric Clinics of North America. 46 (1): 79–87. doi:10.1016/S0031-3955(05)70082-1. PMID 10079791.
- Mayberry RI, Lock E, Kazmi H (May 2002). "Linguistic ability and early language exposure". Nature. 417 (6884): 38. doi:10.1038/417038a. PMID 11986658.
- Johnson JS, Newport EL (June 1991). "Critical period effects on universal properties of language: the status of subjacency in the acquisition of a second language". Cognition. 39 (3): 215–58. doi:10.1016/0010-0277(91)90054-8. PMID 1841034.
- Yoshinaga-Itano C (2003). "From Screening to Early Identification and Intervention: Discovering Predictors to Successful Outcomes for Children With Significant Hearing Loss". Journal of Deaf Studies and Deaf Education. 8 (1): 11–30. doi:10.1093/deafed/8.1.11. PMID 15448044.
- Neville H, Bavelier D (2002). Human brain plasticity: evidence from sensory deprivation and altered language experience. Progress in Brain Research. 138. pp. 177–88. doi:10.1016/S0079-6123(02)38078-6. ISBN 9780444509819. PMID 12432770.
- Muse C, Harrison J, Yoshinaga-Itano C, Grimes A, Brookhouser PE, Epstein S, Buchman C, Mehl A, Vohr B, Moeller MP, Martin P (Apr 2013). "Supplement to the JCIH 2007 Position Statement: Principles and Guidelines for Early Intervention After Confirmation That a Child Is Deaf or Hard of Hearing". Pediatrics. 131 (4). doi:10.1542/peds.2013-0008.
- Childhood hearing loss: strategies for prevention and care. World Health Organization. 2016. ISBN 9789241510325.
- "EHDI Programs". U.S. Centers for Disease Control and Prevention. 2017-11-14.
- "NHS Newborn Hearing Screening Programme Home Page". UK National Health Service.
- "Universal Newborn Hearing Screening Programme". New Zealand National Screening Unit.
- Levêque, Alain; Tognola, Gabriella; Lagasse, Raphaël; Senterre, Christelle; Vos, Bénédicte (2016-06-01). "Organisation of newborn hearing screening programmes in the European Union: widely implemented, differently performed". European Journal of Public Health. 26 (3): 505–510. doi:10.1093/eurpub/ckw020. ISSN 1101-1262.
- "Joint Committee on Infant Hearing". Retrieved 2019-03-01.
- "Newborn Hearing Screening". American Speech-Language-Hearing Association (ASHA). Retrieved 6 March 2019.
- Thompson DC, McPhillips H, Davis RL, Lieu TL, Homer CJ, Helfand M (24 October 2001). "Universal newborn hearing screening: summary of evidence". JAMA. 286 (16): 2000–10. CiteSeerX 10.1.1.599.9440. doi:10.1001/jama.286.16.2000. PMID 11667937.
- Wrightson AS (May 2007). "Universal newborn hearing screening". American Family Physician. 75 (9): 1349–52. PMID 17508530.
- "Hearing Loss at Birth (Congenital Hearing Loss)". American Speech-Language-Hearing Association. Retrieved 2019-03-04.
- "Published Literature on Prevalence of Hearing Loss in Children" (PDF). U.S. Centers for Disease Control and Prevention.
- Barrett TS, White KR (2017). "Trends in hearing loss among adolescents". Pediatrics. 140 (6). doi:10.1542/peds.2017-0619.
- Yoshinaga-Itano C, Sedey AL, Wiggin M, Chung W (2017). "Early Hearing Detection and Vocabulary of Children With Hearing Loss". Pediatrics. 140 (2). doi:10.1542/peds.2016-2964. PMC 5595069.
- Hunter LL, Meinzen-Derr J, Wiley S, Horvath CL, Kothari R, Wexelblatt S (July 2016). "Influence of the WIC Program on Loss to Follow-up for Newborn Hearing Screening". Pediatrics. 138 (1). doi:10.1542/peds.2015-4301. PMC 4925076.
- Seeliger EL, Martin RA, Gromoske AN, Harris, AB (2016). "WIC Participation as a Risk Factor for Loss to Follow-Up in the Wisconsin EHDI System". Journal of Early Hearing Detection and Intervention. 1 (1). doi:10.15142/T3KK5C.
- Cone B, Norrix L (2015). "Measuring the Advantage of Kalman-Weighted Averaging for Auditory Brainstem Response Hearing Evaluation in Infants". American Journal of Audiology. 24 (2). doi:10.1044/2015_AJA-14-0021.
- Ross DS, Holstrum WJ, Gaffney M, Green D, Oyler RF, Gravel JS (Mar 2008). "Hearing Screening and Diagnostic Evaluation of Children With Unilateral and Mild Bilateral Hearing Loss". Trends Amplif. 12 (1). doi:10.1177/1084713807306241. PMC 4111446.
- Chapman DA, Stampfel CC, Bodurtha JN, Dodson KM, Pandya A, Lynch KB, Kirby RS (Dec 2011). "Impact of Co-Occurring Birth Defects on the Timing of Newborn Hearing Screening and Diagnosis". Am J Audiol. 20 (2). doi:10.1044/1059-0889(2011/10-0049).
- Lanzieri TM, Leung J, Caviness AC, Chung W, Flores M, Blum P, Bialek SR, Miller JA, Vinson SS, Turcich MR, Voigt RG (April 2017). "Long-term outcomes of children with symptomatic congenital cytomegalovirus disease". Journal of Perinatology. 37 (7). doi:10.1038/jp.2017.41.
- "Joint Committee on Infant Hearing 1994 position statement". Pediatrics. 95 (1). Jan 1995. PMID 7770297.
- "Newborn and infant hearing screening: Current issues and guiding principles for action" (PDF). World Health Organization. Retrieved 6 March 2019.
- "Recommended Uniform Screening Panel". U.S. Health Resources & Services Administration. Retrieved 2019-03-01.
- "Directors of Speech and Hearing Programs in State Health and Welfare Agencies". Retrieved 2019-03-01.
- "Information About EHDI State Programs". U.S. Centers for Disease Control and Prevention. 2017-11-14. Retrieved 2019-03-05.
- "Identifying infants with hearing loss – United States, 1999–2007". Morbidity and Mortality Weekly Report. 59 (8): 220–3. March 2010. PMID 20203554 – via U.S. Centers for Disease Control Prevention.
- Gaffney M, Eichwald J, Gaffney C, Alam S (September 2014). "Early hearing detection and intervention among infants--hearing screening and follow-up survey, United States, 2005–2006 and 2009–2010". MMWR Supplements. 63 (2): 20–6. PMID 25208254.
- "Summary of 2016 National CDC EHDI Data" (PDF). U.S. Centers for Disease Control and Prevention. May 2018.
- American Academy Of Pediatrics, Joint Committee on Infant Hearing (October 2007). "Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs". Pediatrics. 120 (4): 898–921. doi:10.1542/peds.2007-2333. PMID 17908777.
- Alam S, Gaffney M, Eichwald J (2014). "Improved newborn hearing screening follow-up results in more infants identified". Journal of Public Health Management and Practice. 20 (2): 220–3. doi:10.1097/PHH.0b013e31829d7b57. PMC 4470168. PMID 23803975.
- Downs MP, Sterritt GM (1964). "Identification audiometry for neonates: a preliminary report". Journal of Auditory Research.
- Houston KT, Behl DD, White KR, Forsman I (August 2010). "Federal privacy regulations and the provision of Early Hearing Detection and Intervention programs". Pediatrics. 126 Suppl 1: S28–33. doi:10.1542/peds.2010-0354G. PMID 20679317.
- Russ SA, Dougherty D, Jagadish P (August 2010). "Accelerating evidence into practice for the benefit of children with early hearing loss". Pediatrics. 126 Suppl 1: S7–18. doi:10.1542/peds.2010-0354E. PMID 20679322.
- Russ SA, Hanna D, DesGeorges J, Forsman I (August 2010). "Improving follow-up to newborn hearing screening: a learning-collaborative experience". Pediatrics. 126 (Suppl 1): S59–69. doi:10.1542/peds.2010-0354K. PMID 20679321.
- Shulman S, Besculides M, Saltzman A, Ireys H, White KR, Forsman I (August 2010). "Evaluation of the universal newborn hearing screening and intervention program". Pediatrics. 126 Suppl 1: S19–27. doi:10.1542/peds.2010-0354F. PMID 20679316.
- Wedenberg E (1956). "Auditory tests on newborn infants". Acta Oto-Laryngologica. 46 (5): 446–61. doi:10.3109/00016485609120155. PMID 13361864.
- "Joint Committee on Infant Hearing" (PDF). Retrieved 6 March 2019.
- Mahoney TM, Eichwald JG (May 1987). "The ups and "downs" of high-risk hearing screening: The Utah statewide program". Seminars in Hearing. 8 (2): 155–163. doi:10.1055/s-0028-1091366.
- "Early identification of hearing impairment in infants and young children". NIH Consensus Statement. 11 (1): 1–24. 1993. PMID 8401641.
- Northern JL, Downs MP (2002). "Chapter 1: Hearing and Hearing Loss in Children". Hearing in children. Lippincott Williams & Wilkins. p. 4.
- Walsh, James T. (1991-05-06). "Actions – H.R.2089 – 102nd Congress (1991–1992): Hearing Loss Testing Act of 1991". www.congress.gov. Retrieved 2019-03-01.
- Maxon AB, White KR, Vohr BR, Behrens TR (April 1993). "Using transient evoked otoacoustic emissions for neonatal hearing screening". British Journal of Audiology. 27 (2): 149–53. doi:10.3109/03005369309077906. PMID 8220282.
- Vohr BR, Carty LM, Moore PE, Letourneau K (September 1998). "The Rhode Island Hearing Assessment Program: experience with statewide hearing screening (1993–1996)". The Journal of Pediatrics. 133 (3): 353–7. doi:10.1016/S0022-3476(98)70268-9. PMID 9738715.
- Nelson HD, Bougatsos C, Nygren P (Jul 2008). "Universal newborn hearing screening: systematic review to update the 2001 US Preventive Services Task Force Recommendation". Pediatrics. 122 (1). doi:10.1542/peds.2007-1422.
- CDC (2017-11-14). "Information About EHDI State Programs". Centers for Disease Control and Prevention. Retrieved 2019-03-01.
- 127569 (2016-07-26). "Early Hearing Detection & Intervention (EHDI)". American Academy of Otolaryngology–Head and Neck Surgery. Retrieved 2019-03-01.
- White KR, Forsman I, Eichwald J, Munoz K (April 2010). "The evolution of early hearing detection and intervention programs in the United States". Seminars in Perinatology. 34 (2): 170–9. doi:10.1053/j.semperi.2009.12.009. PMID 20207267.
- "Profiles". IHE International. Retrieved 2019-03-01.
- "Newborn Screening Coding and Terminology Guide". U.S. National Library of Medicine. Retrieved 2019-03-01.
- "Electronic Clinical Quality Measure (eCQM) Standards Landscape" (PDF). HE Quality, Research and Public Health (QRPH) White Paper. IHE International, Inc. 6 February 2018.
- "Early Hearing Detection & Intervention Pediatric Audiology Links to Services (EHDI-PALS)". Retrieved 2019-03-01.
- "HL7 Version 2.6 Implementation Guide: Early Hearing Detection and Intervention (EHDI) Results Release 1". HL7 International. Retrieved 2019-03-01.
Further reading
- "European Consensus Statement on Neonatal Hearing Screening". Audiology. 38 (2): 119. 1999. PMID 10206521.
External links
- Resources on Newborn Hearing Screening by the American Speech-Language-Hearing Association
- Resources on Newborn Hearing Screening by the UK NHS
- Fact Sheet/ Research Portfolio Online Reporting Tools, Newborn Hearing Screening, National Institutes of Health
- European Consensus Statement on Neonatal Hearing Screening 1999.