T-cell receptor
The T-cell receptor (TCR) is a molecule found on the surface of T cells, or T lymphocytes,[1] that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding between TCR and antigen peptides is of relatively low affinity and is degenerate: that is, many TCRs recognize the same antigen peptide and many antigen peptides are recognized by the same TCR.[2]
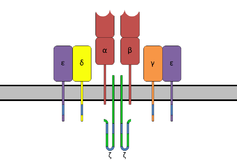 The T-cell receptor complex with TCR-α and TCR-β chains, CD3 and ζ-chain accessory molecules. | |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | TCR_zetazeta | ||||||||
| Pfam | PF11628 | ||||||||
| InterPro | IPR021663 | ||||||||
| OPM superfamily | 166 | ||||||||
| OPM protein | 2hac | ||||||||
| Membranome | 26 | ||||||||
| |||||||||
| T-cell receptor alpha locus | |
|---|---|
| Identifiers | |
| Symbol | TRA |
| Alt. symbols | TCRA, TRA@ |
| NCBI gene | 6955 |
| HGNC | 12027 |
| OMIM | 186880 |
| Other data | |
| Locus | Chr. 14 q11.2 |
| T-cell receptor beta locus | |
|---|---|
| Identifiers | |
| Symbol | TRB |
| Alt. symbols | TCRB, TRB@ |
| NCBI gene | 6957 |
| HGNC | 12155 |
| OMIM | 186930 |
| Other data | |
| Locus | Chr. 7 q34 |
| T-cell receptor delta locus | |
|---|---|
| Identifiers | |
| Symbol | TRD |
| Alt. symbols | TCRD, TRD@, TCRDV1 |
| NCBI gene | 6964 |
| HGNC | 12252 |
| Other data | |
| Locus | Chr. 14 q11.2 |
| T-cell receptor gamma locus | |
|---|---|
| Identifiers | |
| Symbol | TRG |
| Alt. symbols | TCRG, TRG@ |
| NCBI gene | 6965 |
| HGNC | 12271 |
| Other data | |
| Locus | Chr. 7 p14 |
The TCR is composed of two different protein chains (that is, it is a heterodimer). In humans, in 95% of T cells the TCR consists of an alpha (α) chain and a beta (β) chain (encoded by TRA and TRB, respectively), whereas in 5% of T cells the TCR consists of gamma and delta (γ/δ) chains (encoded by TRG and TRD, respectively). This ratio changes during ontogeny and in diseased states (such as leukemia). It also differs between species. Orthologues of the 4 loci have been mapped in various species.[3][4] Each locus can produce a variety of polypeptides with constant and variable regions.[3]
When the TCR engages with antigenic peptide and MHC (peptide/MHC), the T lymphocyte is activated through signal transduction, that is, a series of biochemical events mediated by associated enzymes, co-receptors, specialized adaptor molecules, and activated or released transcription factors.
History of the TCR
In 1984, Tak Wah Mak[5] and Mark M. Davis[6] discovered the human and mouse TCR respectively. These findings allowed the entity and structure of the elusive TCR, known before as the "Holy Grail of Immunology", to be revealed. This allowed scientists from around the world to carry out studies on the TCR, leading to important studies in the fields of CAR-T, Cancer immunotherapy and Checkpoint inhibition.
Structural characteristics of the TCR
The TCR is a disulfide-linked membrane-anchored heterodimeric protein normally consisting of the highly variable alpha (α) and beta (β) chains expressed as part of a complex with the invariant CD3 chain molecules. T cells expressing this receptor are referred to as α:β (or αβ) T cells, though a minority of T cells express an alternate receptor, formed by variable gamma (γ) and delta (δ) chains, referred as γδ T cells.[7]
Each chain is composed of two extracellular domains: Variable (V) region and a Constant (C) region, both of Immunoglobulin superfamily (IgSF) domain forming antiparallel β-sheets. The Constant region is proximal to the cell membrane, followed by a transmembrane region and a short cytoplasmic tail, while the Variable region binds to the peptide/MHC complex.
The variable domain of both the TCR α-chain and β-chain each have three hypervariable or complementarity determining regions (CDRs). There is also an additional area of hypervariability on the β-chain (HV4) that does not normally contact antigen and, therefore, is not considered a CDR.
The residues in these variable domains are located in two regions of the TCR, at the interface of the α- and β-chains and in the β-chain framework region that is thought to be in proximity to the CD3 signal-transduction complex.[8] CDR3 is the main CDR responsible for recognizing processed antigen, although CDR1 of the alpha chain has also been shown to interact with the N-terminal part of the antigenic peptide, whereas CDR1 of the β-chain interacts with the C-terminal part of the peptide.
CDR2 is thought to recognize the MHC. CDR4 of the β-chain is not thought to participate in antigen recognition, but has been shown to interact with superantigens.
The constant domain of the TCR consists of short connecting sequences in which a cysteine residue forms disulfide bonds, which form a link between the two chains.
The TCR is a member of the immunoglobulin superfamily, a large group of proteins involved in binding, recognition, and adhesion; the family is named after antibodies (also called immunoglobulins). The TCR is similar to a half-antibody consisting of a single heavy and single light chain, except the heavy chain is without its crystallisable fraction (Fc). The two subunits of TCR are twisted together. Whereas the antibody uses its Fc region to bind to Fc Receptors on leukocytes, TCR is already docked onto the cell membrane. However, it is not able to mediate signal transduction itself due to its short cytoplasmic tail, so TCR still requires CD3 and zeta to carry out the signal transduction in its place, just as antibodies require binding to FcRs to initiate signal transduction. In this way the MHC-TCR-CD3 interaction for T cells is functionally similar to the antigen(Ag)-immunoglobulin(Ig)-FcR interaction for myeloid leukocytes, and Ag-Ig-CD79 interaction for B cells.
Generation of the TCR diversity
The generation of TCR diversity is similar to that for antibodies and B cell antigen receptors. It arises mainly from genetic recombination of the DNA encoded segments in individual somatic T cells by somatic V(D)J recombination using RAG1 and RAG2 recombinases. Unlike immunoglobulins, however, TCR genes do not undergo somatic hypermutation, and T cells do not express activation-induced cytidine deaminase(AID). The recombination process that creates diversity in BCR (antibodies) and TCR is unique to lymphocytes (T and B cells) during the early stages of their development in primary lymphoid organs (thymus for T cells, bone marrow for B cells).
Each recombined TCR possess unique antigen specificity, determined by the structure of the antigen-binding site formed by the α and β chains in case of αβ T cells or γ and δ chains on case of γδ T cells.[9]
- The TCR alpha chain is generated by VJ recombination, whereas the beta chain is generated by VDJ recombination (both involving a random joining of gene segments to generate the complete TCR chain).
- Likewise, generation of the TCR gamma chain involves VJ recombination, whereas generation of the TCR delta chain occurs by VDJ recombination.
The intersection of these specific regions (V and J for the alpha or gamma chain; V, D, and J for the beta or delta chain) corresponds to the CDR3 region that is important for peptide/MHC recognition (see above).
It is the unique combination of the segments at this region, along with palindromic and random nucleotide additions (respectively termed "P-" and "N-"), which accounts for the even greater diversity of T-cell receptor specificity for processed antigenic peptides.
Later during development, individual CDR loops of TCR can be re-edited in the periphery outside thymus by reactivation of recombinases using a process termed TCR revision (editing) and change its antigenic specificity.
The TCR complex
In the plasma membrane the TCR receptor chains α and β associate with six additional adaptor proteins to form an octameric complex. The complex contains both α and β chains, forming the ligand-binding site, and the signaling modules CD3δ, CD3γ, CD3ε and CD3ζ in the stoichiometry TCR α β - CD3εγ - CD3εδ - CD3ζζ. Charged residues in the transmembrane domain of each subunit form polar interactions allowing a correct and stable assembly of the complex.[10] The cytoplasmic tail of the TCR is extremely short, hence the CD3 adaptor proteins contain the signalling motifs needed for propagating the signal from the triggered TCR into the cell. The signalling motifs involved in TCR signalling are tyrosine residues in the cytoplasmic tail of these adaptor proteins that can be phosphorylated in the event of TCR-pMHC binding. The tyrosine residues reside in a specific amino acid sequence of the signature Yxx(L/I)x6-8Yxx(L/I), where Y, L, I indicate tyrosine, leucine and isoleucine residues, x denotes any amino acids, the subscript 6-8 indicates a sequence of 6 to 8 amino acids in length. This motif is very common in activator receptors of the non-catalytic tyrosine-phosphorylated receptor (NTR) family and is referred to as immunoreceptor tyrosine-based activation motif (ITAM).[11] CD3δ, CD3γ and CD3ε each contain a single ITAM, while CD3ζ contains three ITAMs. In total the TCR complex contains 10 ITAMs.[10] Phosphorylated ITAMs act as binding site for SH2-domains of additionally recruited proteins.
Antigen discrimination
Each T cell expresses clonal TCRs which recognize a specific peptide loaded on a MHC molecule (pMHC), either on MHC class II on the surface of antigen presenting cells or MHC class I on any other cell type.[12] A unique feature of T cells is their ability to discriminate between peptides derived from healthy, endogenous cells and peptides from foreign or abnormal (e.g. infected or cancerous) cells in the body.[13] Antigen presenting cells do not discriminate between self and foreign peptides and typically express a large number of self derived pMHC on their cell surface and only a few copies of any foreign pMHC. For example, it has been shown that cells infected with HIV have only 8-46 HIV specific pMHCs next to 100000 total pMHC per cell.[14][15]
Because T cells undergo positive selection in the thymus there is a non-negligible affinity between self pMHC and the TCR, nevertheless, the T cell receptor signalling should not be activated by self pMHC such that endogeneous, healthy cells are ignored by T cells. However, when these very same cells contain even minute quantities of pathogen derived pMHC, T cells must get activated and initiate immune responses. The ability of T cells to ignore healthy cells but respond when these same cells express a small number of foreign pMHC is known as antigen discrimination.[16][17]
To do so, T cells have a very high degree of antigen specificity, despite of fact that the affinity to the peptide/MHC ligand is rather low in comparison to other receptor types.[18] The affinity, given as the dissociation constant (Kd), between a TCR and a pMHC was determined by surface plasmon resonance (SPR) to be in the range of 1-100 μM, with an association rate (kon) of 1000 -10000 M−1 s−1 and a dissociation rate (koff) of 0.01 -0.1 s−1.[19] In comparison, cytokines have an affinity of KD = 10-600 pM to their receptor.[20] It has been shown that even a single amino acid change in the presented peptide that affects the affinity of the pMHC to the TCR reduces the T cell response and cannot be compensated by a higher pMHC concentration.[21] A negative correlation between the dissociation rate of the pMHC-TCR complex and the strength of the T cell response has been observed.[22] That means, pMHC that bind the TCR for a longer time initiate a stronger activation of the T cell. Furthermore, T cells are very sensitive. Interaction with a single pMHC is enough to trigger activation.[23] Also, the decision whether a T cell response to an antigen is made fast. T cells rapidly scan pMHC on a antigen presenting cell to increase the change of finding a specific pMHC. On average, T cell encounter 20 APCs per hour.[24]
Different models for the molecular mechanisms that underlie this highly specific and highly sensitive process of antigen discrimination have been proposed. The occupational model simply suggests that the TCR response is proportional to the number of pMHC bound to the receptor. Given this model, a shorter lifetime of a peptide can be compensated by higher concentration such that the maximum response of the T cell stays the same. However, this cannot be seen in experiments and the model has been widely rejected.[22] The most accepted view is that the TCR engages in kinetic proofreading. The kinetic proofreading model proposes that a signal is not directly produced upon binding but a series of intermediate steps insure a time delay between binding and signal output. Such intermediate “proofreading” steps can be multiple rounds of tyrosine phosphorylation. These steps require energy and therefore do not happen spontaneously, only when the receptor is bound to its ligand. This way only ligands with high affinity that bind the TCR for a long enough time can initiate a signal. All intermediate steps are reversible, such that upon ligand dissociation the receptor reverts to its original unphosphorylated state before a new ligand binds.[25] This model predicts that maximum response of T cells decreases for pMHC with shorter lifetime. Experiments have confirmed this model.[22] However, the basic kinetic proofreading model has a trade-off between sensitivity and specificity. Increasing the number of proofreading steps increases the specificity but lowers the sensitivity of the receptor. The model is therefore not sufficient to explain the high sensitivity and specificity of TCRs that have been observed. (Altan Bonnet2005) Multiple models that extend the kinetic proofreading model have been proposed, but evidence for the models is still controversial.[13][26][27]
The antigen sensitivity is higher in antigen-experienced T cells than in naive T cells. Naive T cells pass through the process of functional avidity maturation with no change in affinity. It is based on the fact that effector and memory (antigen-experienced) T cell are less dependent on costimulatory signals and higher antigen concentration than naive T cell.[28]
Signaling pathway
The essential function of the TCR complex is to identify specific bound antigen derived from a potentially harmful pathogen and elicit a distinct and critical response. At the same time it has to ignore any self-antigen and tolerate harmless antigens such as food antigens. The signal transduction mechanism by which a T cell elicits this response upon contact with its unique antigen is termed T-cell activation. Upon binding to pMHC, the TCR initiates a signalling cascade, involving transcription factor activation and cytoskeletal remodelling resulting in T cell activation. Active T cells secrete cytokines, undergo rapid proliferation, have cytotoxic activity and differentiate into effector and memory cells. When the TCR is triggered, T cells form an immunological synapse allowing them to stay in contact with the antigen presenting cell for several hours.[29] On a population level, T cell activation depends on the strength of TCR stimulation, the dose–response curve of ligand to cytokine production is sigmoidal. However, T cell activation on a single cell level can be characterised by a digital switch-like response, meaning the T cell is fully activated if the stimulus is higher than a given threshold, otherwise the T cell stay in its non-activated state. There is no intermediate activation state. The robust sigmoid dose-response curve on population level results from individual T cells having slightly different thresholds.[21] There are myriad molecules involved in the complex biochemical process (called trans-membrane signaling) by which T-cell activation occurs.
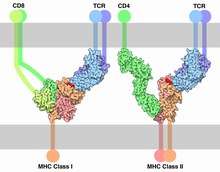
TCR co-receptors
The signal from the T-cell complex is enhanced by simultaneous binding of the MHC molecules by a specific co-receptor.
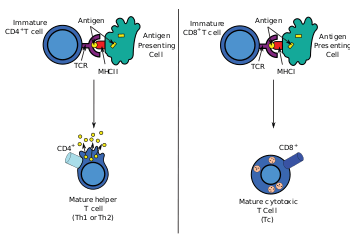
- On helper T cells and regulatory T cells, this co-receptor is CD4 that is specific for MHC class II.
- On cytotoxic T cells, this co-receptor is CD8 that is specific for MHC class I.
Extracellularly, the TCR co-receptor defines the specificity of the TCR to MHC class I or II molecule, and increases binding affinity of TCR to MHC to prolong the cell-cell interaction between the antigen-presenting cell and the T cell.
Intracellularly, the TCR co-receptor recruits essential molecules (e.g., LCK) involved in the signaling of the activated T lymphocyte to facilitate the CD3 signal transduction mechanism.
Associated molecules of the TCR complex involved in T-cell activation
The most common mechanism for activation and regulation of molecules beneath the lipid bilayer is via reversible tyrosine phosphorylation by protein kinase/phosphatase. T cells utilise the Src family kinases in transmembrane signalling largely to phosphorylate tyrosines that are part of immunoreceptor tyrosine-based activation motifs (ITAM) in intracellular parts of CD3 and ζ chains.[30]
Early signaling steps implicate the following kinases and phosphatases after TCR triggering:
- Lck – a Src family kinase associated with the intracellular tail of CD4 that phosphorylates CD3 and ζ ITAMs of the TCR complex
- FYN – a Src family kinase that phosphorylates CD3 and ζ ITAMs
- CD45 – a transmembrane protein whose intracellular tail functions as a tyrosine phosphatase that activates Src family kinases
- Zap70 – a Syk family kinase that binds to ITAM sequences upon tyrosine phosphorylation by Lck and Fyn, and phosphorylates LAT
When a T-cell receptor is activated by contact with a peptide:MHC complex, CD45 dephosphorylates inhibitory tyrosine of membrane-localized Src family kinases Fyn and Lck, previously recruited and activated by CD4 or CD8 coreceptors. Activated Fyn and Lck phosphorylates ITAMs on the CD3 and ζ chains. This allows cytoplasmic kinases of the Syk family (ZAP-70) to bind to the ITAM and activated ZAP-70 phosphorylates tyrosines on the adaptor protein LAT, which then attracts PLC-γ. Other downstream pathways are triggered as well (MAPK, NF-κB, NFAT) which results in gene transcription in the nucleus.[31]
See also
- ImmTAC
- Co-stimulation
- MHC multimer
References
- Kindt TJ, Goldsby RA, Osborne BA, Kuby J (2007). Kuby immunology. Macmillan. pp. 223–. ISBN 978-1-4292-0211-4. Retrieved 28 November 2010.
- Sewell AK (September 2012). "Why must T cells be cross-reactive?". Nature Reviews. Immunology. 12 (9): 669–77. doi:10.1038/nri3279. PMID 22918468.
- Glusman G, Rowen L, Lee I, Boysen C, Roach JC, Smit AF, et al. (September 2001). "Comparative genomics of the human and mouse T cell receptor loci". Immunity. 15 (3): 337–49. doi:10.1016/s1074-7613(01)00200-x. PMID 11567625.
- Deakin JE, Parra ZE, Graves JA, Miller RD (2006). "Physical mapping of T cell receptor loci (TRA@, TRB@, TRD@ and TRG@) in the opossum (Monodelphis domestica)". Cytogenetic and Genome Research. 112 (3–4): 342K. doi:10.1159/000089901. PMID 16484802.
- Yanagi Y, Yoshikai Y, Leggett K, Clark SP, Aleksander I, Mak TW (8 March 1984). "A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains". Nature. 308 (5955): 145–9. Bibcode:1984Natur.308..145Y. doi:10.1038/308145a0. PMID 6336315.
- Hedrick SM, Cohen DI, Nielsen EA, Davis MM (8 March 1984). "Isolation of cDNA clones encoding T cell-specific membrane-associated proteins". Nature. 308 (5955): 149–53. Bibcode:1984Natur.308..149H. doi:10.1038/308149a0. PMID 6199676.
- Janeway Jr CA, Travers P, Walport M, et al. (2001). Immunobiology: The Immune System in Health and Disease. 5th edition. Glossary: Garland Science.
- Kieke MC, Shusta EV, Boder ET, Teyton L, Wittrup KD, Kranz DM (May 1999). "Selection of functional T cell receptor mutants from a yeast surface-display library". Proceedings of the National Academy of Sciences of the United States of America. 96 (10): 5651–6. Bibcode:1999PNAS...96.5651K. doi:10.1073/pnas.96.10.5651. PMC 21915. PMID 10318939.
- Janeway CA, Travers P, Walport M, et al. (2001). Immunobiology: The Immune System in Health and Disease (5th ed.). Chapter 4, The Generation of Lymphocyte Antigen Receptors: Garland Science.
- Call ME, Pyrdol J, Wiedmann M, Wucherpfennig KW (December 2002). "The organizing principle in the formation of the T cell receptor-CD3 complex". Cell. 111 (7): 967–79. doi:10.1016/s0092-8674(02)01194-7. PMC 3420808. PMID 12507424.
- Dushek O, Goyette J, van der Merwe PA (November 2012). "Non-catalytic tyrosine-phosphorylated receptors". Immunological Reviews. 250 (1): 258–76. doi:10.1111/imr.12008. PMID 23046135.
- Smith-Garvin JE, Koretzky GA, Jordan MS (2009). "T cell activation". Annual Review of Immunology. 27: 591–619. doi:10.1146/annurev.immunol.021908.132706. PMC 2740335. PMID 19132916.
- Feinerman O, Germain RN, Altan-Bonnet G (February 2008). "Quantitative challenges in understanding ligand discrimination by alphabeta T cells". Molecular Immunology. 45 (3): 619–31. doi:10.1016/j.molimm.2007.03.028. PMC 2131735. PMID 17825415.
- Yang H, Buisson S, Bossi G, Wallace Z, Hancock G, So C, et al. (November 2016). "Elimination of Latently HIV-infected Cells from Antiretroviral Therapy-suppressed Subjects by Engineered Immune-mobilizing T-cell Receptors". Molecular Therapy. 24 (11): 1913–1925. doi:10.1038/mt.2016.114. PMC 5154472. PMID 27401039.
- Blum JS, Wearsch PA, Cresswell P (2013). "Pathways of antigen processing". Annual Review of Immunology. 31: 443–73. doi:10.1146/annurev-immunol-032712-095910. PMC 4026165. PMID 23298205.
- Evavold BD, Allen PM (May 1991). "Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand". Science. 252 (5010): 1308–10. Bibcode:1991Sci...252.1308E. doi:10.1126/science.1833816. PMID 1833816.
- Kersh GJ, Allen PM (October 1996). "Structural basis for T cell recognition of altered peptide ligands: a single T cell receptor can productively recognize a large continuum of related ligands". The Journal of Experimental Medicine. 184 (4): 1259–68. doi:10.1084/jem.184.4.1259. PMC 2192852. PMID 8879197.
- Donermeyer DL, Weber KS, Kranz DM, Allen PM (November 2006). "The study of high-affinity TCRs reveals duality in T cell recognition of antigen: specificity and degeneracy". Journal of Immunology. 177 (10): 6911–9. doi:10.4049/jimmunol.177.10.6911. PMID 17082606.
- Cole DK, Pumphrey NJ, Boulter JM, Sami M, Bell JI, Gostick E, et al. (May 2007). "Human TCR-binding affinity is governed by MHC class restriction". Journal of Immunology. 178 (9): 5727–34. doi:10.4049/jimmunol.178.9.5727. PMID 17442956.
- Whitty A, Raskin N, Olson DL, Borysenko CW, Ambrose CM, Benjamin CD, Burkly LC (October 1998). "Interaction affinity between cytokine receptor components on the cell surface". Proceedings of the National Academy of Sciences of the United States of America. 95 (22): 13165–70. Bibcode:1998PNAS...9513165W. doi:10.1073/pnas.95.22.13165. PMC 23746. PMID 9789059.
- Altan-Bonnet G, Germain RN (November 2005). "Modeling T cell antigen discrimination based on feedback control of digital ERK responses". PLoS Biology. 3 (11): e356. doi:10.1371/journal.pbio.0030356. PMC 1262625. PMID 16231973.
- Dushek O, Aleksic M, Wheeler RJ, Zhang H, Cordoba SP, Peng YC, et al. (June 2011). "Antigen potency and maximal efficacy reveal a mechanism of efficient T cell activation". Science Signaling. 4 (176): ra39. doi:10.1126/scisignal.2001430. PMC 4143974. PMID 21653229.
- Huang J, Brameshuber M, Zeng X, Xie J, Li QJ, Chien YH, et al. (November 2013). "A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells". Immunity. 39 (5): 846–57. doi:10.1016/j.immuni.2013.08.036. PMC 3846396. PMID 24120362.
- Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I (January 2004). "T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node". Proceedings of the National Academy of Sciences of the United States of America. 101 (4): 998–1003. Bibcode:2004PNAS..101..998M. doi:10.1073/pnas.0306407101. PMC 327133. PMID 14722354.
- McKeithan TW (May 1995). "Kinetic proofreading in T-cell receptor signal transduction". Proceedings of the National Academy of Sciences of the United States of America. 92 (11): 5042–6. Bibcode:1995PNAS...92.5042M. doi:10.1073/pnas.92.11.5042. PMC 41844. PMID 7761445.
- Dushek O, van der Merwe PA (April 2014). "An induced rebinding model of antigen discrimination". Trends in Immunology. 35 (4): 153–8. doi:10.1016/j.it.2014.02.002. PMC 3989030. PMID 24636916.
- Lever M, Maini PK, van der Merwe PA, Dushek O (September 2014). "Phenotypic models of T cell activation". Nature Reviews. Immunology. 14 (9): 619–29. doi:10.1038/nri3728. PMID 25145757.
- von Essen MR, Kongsbak M, Geisler C (2012). "Mechanisms behind functional avidity maturation in T cells". Clinical & Developmental Immunology. 2012: 163453. doi:10.1155/2012/163453. PMC 3351025. PMID 22611418.
- Janeway's immunobiology (Ninthition ed.). ISBN 0815345518.
- Abram CL, Lowell CA (March 2007). "The expanding role for ITAM-based signaling pathways in immune cells". Science's STKE. 2007 (377): re2. doi:10.1126/stke.3772007re2. PMID 17356173.
- Parham P (2009). The Immune System. New York: Garland Science. pp. 22–223. ISBN 978-0-8153-4146-8.
External links
| Wikimedia Commons has media related to T-cell antigen receptors. |
- T-cell Group – Cardiff University
- UMich Orientation of Proteins in Membranes protein/pdbid-2hac – Zeta-zeta dimer of T cell receptor
- T-Cell+Receptor at the US National Library of Medicine Medical Subject Headings (MeSH)