Strømme syndrome
Strømme syndrome is a very rare autosomal recessive genetic condition characterised by intestinal atresia (in which part of the intestine is missing), eye abnormalities and microcephaly. The intestinal atresia is of the rare "apple-peel" type, in which the remaining intestine twists around the mesenteric arteries. The front third of the eye is typically underdeveloped, and there is usually moderate developmental delay. Less common features include an atrial septal defect, increased muscle tone or skeletal abnormalities.[2][3] Facial features may include large, low-set ears, a small jaw, a large mouth, epicanthic folds or fine, sparse hair.[3][2][5]
| Strømme syndrome | |
|---|---|
| Other names | Stromme syndrome, apple-peel intestinal atresia–ocular anomalies–microcephaly syndrome,[1] jejunal atresia–microcephaly–ocular anomalies syndrome,[1] apple peel syndrome with microcephaly and ocular anomalies,[2] jejunal atresia with microcephaly and ocular anomalies,[2] (formerly) primary ciliary dyskinesia 31 (CILD31)[2] |
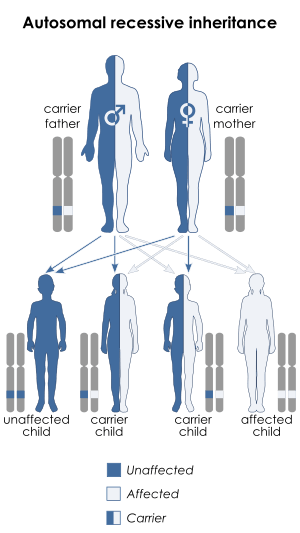 | |
| This condition is inherited in an autosomal recessive manner. | |
| Pronunciation |
|
| Specialty | Medical genetics |
| Symptoms | Apple-peel intestinal atresia, underdeveloped eyes, microcephaly with developmental delay[2][3] |
| Causes | Genetic (autosomal recessive mutation in CENPF)[3][2] |
| Diagnostic method | Based on symptoms, genetic testing[4] |
| Prognosis | Not yet certain. Good for most, though perinatal mortality possible in the most severe cases.[3][4] |
| Frequency | Not yet known. Around 13 individuals diagnosed as of 2017.[2] |
The syndrome is caused by mutations in both copies of the CENPF gene, which codes for centromere protein F.[3][2] This protein is involved in cell division, in which it forms part of a protein complex known as a kinetochore that allows cell microtubules to attach to chromosomes to pull them apart into the new cells, which it does specifically for the centromere region of the chromosome. It also helps coordinate microtubules to form cilia, small cell protrusions involved in cell signalling, migration, differentiation and growth. Mutations in CENPF therefore result in some developmental processes being disrupted or not completed, and the syndrome can be classified as a ciliopathy.[2][6][7] The syndrome is typically diagnosed based on the symptoms, but genetic testing provides a full confirmation.[4][7]
Treatment centres around the symptoms. The intestinal atresia is usually surgically correctable in infancy with anastomosis.[3] The prognosis is not yet certain. Patients who have survived birth and infancy (the majority) have continued to live through childhood and adolescence, but a large minority with the most severe cases have died before or shortly after birth.[3][2][4]
The prevalence is not yet known. As of 2017, around 13 individuals had been diagnosed.[2] The syndrome was first identified based on symptoms in two siblings by Norwegian paediatrician Petter Strømme and his associates in 1993.[2][8] It was named after him in a 2008 study by van Bever et al. describing another patient.[2][9] In 2015, mutations in CENPF were first identified as pathogenic,[2][6] and in 2016 a genetic analysis of Strømme's original two siblings found that both had mutations in both of their copies of CENPF, establishing it as the cause of the syndrome.[2][7]
Signs and symptoms
Patients are typically born with intestinal atresia, in which parts of the intestine are narrow or missing, leading to neonatal bowel obstruction.[3] The intestinal atresia is of the "apple-peel" type, a rare type in which the remaining portion of the intestine twists around the mesenteric arteries, and usually affects the jejunum.[3][2] Often, much of the bowel is missing in this form of atresia.[9] There can sometimes also be intestinal malrotation.[3][2][8][10] At least two patients in literature have avoided this symptom, one of which had a sibling with the same mutations who did not.[6][11]
The eyes are often smaller and underdeveloped, usually more severely in one eye than the other.[2] This can manifest as colobomas (holes) in the iris, cataracts, opacity of the cornea (leukoma), sclerocornea (in which the white of the eye blends into the cornea), a small cornea (microcornea) and synechia (in which the iris adheres to the cornea or lens).[3] This underdevelopment of the front of the eye, known as anterior segment dysgenesis (which includes Peters' anomaly), can lead to an increased risk of glaucoma from high intraocular pressure, due to impaired fluid drainage, though this hasn't yet been reported in any of the patients.[3] There may also be crossing of the eyes (esotropia),[8] and less commonly there may be twisted retinal blood vessels or optic nerve hypoplasia.[3][7] The eye anomalies can result in an inability to focus (astigmatism) as well as amblyopia, in which the brain begins to fail to process input from the weaker eye.[7]
Patients usually have microcephaly.[3][2] Most often, the brain structures are normal, but sometimes there has been pachygyria (fewer ridges in the brain) or lissencephaly (shallower ridges).[9][11] Developmental delay is usually present. It has usually been moderate-to-severe, but in some cases it has been mild.[3] A few patients have had increased muscle tone.[2][5][10] One patient had cortical heterotopia, which is reflective of impaired neuronal cell migration during neural development.[12]
Physical features are variable but usually include short stature, large, low-set ears, a high nasal bridge, a small jaw and a large mouth.[2][11][6] Some patients have had epicanthal folds or fine, sparse hair.[5]
A minority of patients have been born with an atrial septal defect, a type of congenital heart defect.[2][11] One patient with a more severe presentation had hip dysplasia, leading to dislocation.[5]
The severity is variable, and some individuals with mutations in both copies of CENPF have not survived to term. In two of these individuals, who were siblings, there were additional symptoms such as underdeveloped kidneys and ureters, leading to a build-up of urine called hydronephrosis, as well as polydactyly of the thumb, flattened vertebrae (platyspondyly), a sternal cleft and a patent foramen ovale (a type of atrial septal defect). There was also reduced heart muscle tissue (myocardium) and abnormally small heart muscle cells (cardiomyocytes). The intestinal atresia, which also included duodenal atresia, and intestinal malrotation were also more severe.[2][7] Other individuals who did not survive to term had features such as hydrocephalus, cerebellar hypoplasia, agenesis of the corpus callosum and cleft palate in addition.[2][6] Hydrocephalus also occurred in one living patient 9 months after birth[2][5] and in zebrafish in which the CENPF genes were experimentally knocked out.[2][6]
Cause
Strømme syndrome is caused by mutations in both copies of the CENPF gene, located on the long arm of chromosome 1.[3][2] CENPF codes for centromere protein F. Centromere proteins are involved in the separation of chromosomes during cell division. This is through forming part of kinetochores, which are large superstructures of proteins which allow the chromosomes (in the dividing form, known as chromatids) to attach to microtubules in the cell (forming what is called the spindle apparatus) in order for the microtubules to pull them apart in the process of dividing the cell. They do this specifically for the centromere, in the centre of the chromosome. Mutations in this gene therefore lead to impaired cell division during early development, which results in some developmental processes being disrupted or not completed.[6][7]
Microtubules are protein structures which serve as the cell's skeleton, and they are made by the centrosome, which contains a pair of cylindrical centrioles at right-angles to each other. Microtubules are also responsible for forming cellular projections called axonemes, which form the core of cellular cilia. A cilium is a hair-like projection on a cell, most of which are involved in cell signalling for cell migration, differentiation and growth. CENPF appears to localise at the end of centrioles during cell division, indicating that it has a role in orienting microtubules to project correctly to form cilia. Mutations in CENPF disrupt this ability to form cilia. Cilia have been found to be fewer in number and shorter when CENPF is mutated, and this affects multiple developmental processes. This means that Strømme syndrome falls under the classification of diseases known as ciliopathies.[2][6]
Mutations that have been identified in CENPF have been mostly nonsense mutations, which result in the protein being cut short and usually non-functional as a result, but frameshift and splice-site mutations have also been identified. Several of the nonsense mutations that have led to this syndrome have been in exon 12 of the gene (out of 20), but mutations in other exons have been identified.[7][6] Severity and symptoms of the syndrome have been variable regardless of the type of mutation but generally consistent within families, suggesting the severity may depend on the presence of mutations in other genes (epistasis).[11]
It has been suggested that an interaction between CENPF and NDE1, which causes microlissencephaly when mutated, explains the microcephaly in Strømme syndrome.[11][6]
Diagnosis
Diagnosis is typically achieved by observation of symptoms, however genetic testing provides a full confirmation. Methods include whole exome sequencing and Sanger sequencing[2][11] as well as panel testing, which involves sequencing a selection of potential genes involved.[4] Brain MRI scans can reveal any brain anomalies that could be associated with the syndrome.[4] The microcephaly, intestinal atresia and some of the eye abnormalities are observable on prenatal ultrasound.[4][2]
Once a family has been identified as being carriers for mutated CENPF genes, prenatal diagnosis and preimplantation genetic diagnosis can be offered for future conceptions.[4]
Treatment
Treatment targets the symptoms. The intestinal atresia is usually surgically correctable in infancy with anastomosis, however no eye surgery treatment has been reported.[3] Van Bever et al. recommended monitoring patients for glaucoma.[13]
Prognosis
The prognosis is not yet certain. The majority of patients have survived birth and infancy, and these have continued to live through childhood and adolescence. A large minority with the most severe presentations, however, have died before birth or shortly after.[4][7][6] The oldest known patients, Strømme's original two siblings, who had a mild-to-moderate presentation, were in their twenties and in employment as of 2016.[2][7]
Epidemiology
The prevalence of the syndrome is not yet known. As of 2017, around 13 individuals had been diagnosed.[2]
History
The condition was first identified in 1993 when Petter Strømme et al. observed two infant siblings with microcephaly and eye abnormalities alongside apple-peel intestinal atresia at Rogaland Central Hospital in Stavanger, Norway and proposed that it constituted a new syndrome.[2][8] Later studies by Slee and Goldblatt (1996),[5] Shanske et al. (2002),[12] Bellini et al. (2002)[10] and others observed other patients with similar symptoms who appeared to have the syndrome.[2]
In 2008, van Bever et al. proposed that the syndrome be named after Strømme, after encountering another patient who seemed to have the syndrome.[2][9]
In 2015, Waters et al. conducted a genetic analysis on a UK family in which four foetuses had miscarried with symptoms of a ciliopathy. They found that the foetuses had mutations in both copies of CENPF. They subsequently analysed a cohort of 1,000 patients with microcephaly and found that one of the patients had mutations in both copies of CENPF. This patient's learning delay was mild-to-moderate, and she did not have any issues with her other bodily systems. This confirmed that mutations in CENPF are pathogenic for the first time.[2][6]
In 2016, Filges et al. followed up with Strømme's original two siblings and found using whole exome sequencing that they both had mutations in both of their copies of the gene CENPF, establishing mutations in CENPF as the cause of Strømme syndrome.[2][7]
Notable cases
- Ruby Ardolf (born 2004) of Minnesota (US), and diagnosed with Strømme syndrome, appeared in an Instagram video answering questions from her mother Angela. This video went viral, gaining over 500,000 views in a week.[14] Angela manages a website, online store, and YouTube channel for her daughter, with over 100,000 subscribers as of October 2019.[15]
References
- "Orphanet: Stromme syndrome". www.orpha.net. Retrieved 8 December 2019.
- "OMIM Entry - # 243605 - STROMME SYNDROME; STROMS". www.omim.org. Retrieved 27 September 2018.
- "Strømme Syndrome | Hereditary Ocular Diseases". disorders.eyes.arizona.edu. Retrieved 27 September 2018.
- Filges, Isabel; Stromme, Petter (5 September 2019). "CUGC for Stromme syndrome and CENPF-related disorders". European Journal of Human Genetics: 1–5. doi:10.1038/s41431-019-0498-y. ISSN 1476-5438.
- Slee, J.; Goldblatt, J. (October 1996). "Further evidence for a syndrome of "apple peel" intestinal atresia, ocular anomalies and microcephaly". Clinical Genetics. 50 (4): 260–262. doi:10.1111/j.1399-0004.1996.tb02640.x. ISSN 0009-9163. PMID 9001813.
- Waters, Aoife M.; Asfahani, Rowan; Carroll, Paula; Bicknell, Louise; Lescai, Francesco; Bright, Alison; Chanudet, Estelle; Brooks, Anthony; Christou-Savina, Sonja; Osman, Guled; Walsh, Patrick (March 2015). "The kinetochore protein, CENPF, is mutated in human ciliopathy and microcephaly phenotypes". Journal of Medical Genetics. 52 (3): 147–156. doi:10.1136/jmedgenet-2014-102691. ISSN 1468-6244. PMC 4345935. PMID 25564561.
- Filges, Isabel; Bruder, Elisabeth; Brandal, Kristin; Meier, Stephanie; Undlien, Dag Erik; Waage, Trine Rygvold; Hoesli, Irene; Schubach, Max; de Beer, Tjaart; Sheng, Ying; Hoeller, Sylvia (April 2016). "Strømme Syndrome Is a Ciliary Disorder Caused by Mutations in CENPF". Human Mutation. 37 (4): 359–363. doi:10.1002/humu.22960. ISSN 1098-1004. PMID 26820108.
- Strømme, P.; Dahl, E.; Flage, T.; Stene-Johansen, H. (October 1993). "Apple peel intestinal atresia in siblings with ocular anomalies and microcephaly". Clinical Genetics. 44 (4): 208–210. doi:10.1111/j.1399-0004.1993.tb03881.x. ISSN 0009-9163. PMID 8261651.
- van Bever, Yolande; van Hest, Liselotte; Wolfs, Roger; Tibboel, Dick; van den Hoonaard, Thelma L.; Gischler, Saskia J. (15 February 2008). "Exclusion of a PAX6, FOXC1, PITX2, and MYCN mutation in another patient with apple peel intestinal atresia, ocular anomalies and microcephaly and review of the literature". American Journal of Medical Genetics. Part A. 146A (4): 500–504. doi:10.1002/ajmg.a.32169. ISSN 1552-4833. PMID 18203155.
- Bellini, Carlo; Mazzella, Massimo; Arioni, Cesare; Fondelli, Maria Paola; Serra, Giovanni (15 June 2002). ""Apple-peel" intestinal atresia, ocular anomalies, and microcephaly syndrome: brain magnetic resonance imaging study". American Journal of Medical Genetics. 110 (2): 176–178. doi:10.1002/ajmg.10392. ISSN 0148-7299. PMID 12116257.
- Ozkinay, Ferda; Atik, Tahir; Isik, Esra; Gormez, Zeliha; Sagiroglu, Mahmut; Sahin, Ozlem Atan; Corduk, Nergul; Onay, Huseyin (June 2017). "A further family of Stromme syndrome carrying CENPF mutation". American Journal of Medical Genetics. Part A. 173 (6): 1668–1672. doi:10.1002/ajmg.a.38173. ISSN 1552-4833. PMID 28407396.
- Shanske, Alan L.; Gurland, Judith E.; Mbekeani, Joyce N.; Bello, Jacqueline A.; Campbell, Deborah; Kleinhaus, Sylvain (January 2002). "Possible new syndrome of microcephaly with cortical migration defects, Peters anomaly and multiple intestinal atresias: a multiple vascular disruption syndrome". Clinical Dysmorphology. 11 (1): 67–69. doi:10.1097/00019605-200201000-00014. ISSN 0962-8827. PMID 11822709.
- van Bever, Yolande; van Hest, Liselotte; Wolfs, Roger; Tibboel, Dick; van den Hoonaard, Thelma L.; Gischler, Saskia J. (15 February 2008). "Exclusion of a PAX6, FOXC1, PITX2, and MYCN mutation in another patient with apple peel intestinal atresia, ocular anomalies and microcephaly and review of the literature". American Journal of Medical Genetics. Part A. 146A (4): 500–504. doi:10.1002/ajmg.a.32169. ISSN 1552-4833. PMID 18203155.
- "Lakeville Mom, Daughter Go Viral On Instagram". CBS Minnesota. 24 May 2017. Retrieved 16 December 2019.
- Chiu, Jessica. "On YouTube, people with disabilities create content to show and normalize their experiences". Washington Post. Retrieved 16 December 2019.
External links
| Classification | |
|---|---|
| External resources |
|