SEP-363856
SEP-363856 (also known as SEP-856)[1] is an investigational antipsychotic that is undergoing clinical trials for the treatment of schizophrenia and Parkinson's disease psychosis.[2] The medication is being developed by the pharmaceutical companies Sunovion Pharmaceuticals and PsychoGenics Inc.[2]
 | |
| Names | |
|---|---|
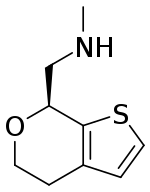
| IUPAC name
(7S)-4,7-Dihydro-N-methyl-5H-thieno[2,3-c]pyran-7-methanamine | |
| Other names
SEP-856 | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C9H13NOS |
| Molar mass | 183.27 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Adverse effects
The adverse effect profile of SEP-363856 differs from that of other antipsychotics because its mechanism of action does not involve antagonism of dopamine receptors in the brain, which is responsible for the drug-induced movement disorders (like akathisia) that may occur with those agents.[3] Some adverse events reported in preliminary clinical trials are somnolence, agitation, nausea, diarrhea, and dyspepsia.[3]
Pharmacology
Mechanism of action
The mechanism of action of SEP-363856 in the treatment of schizophrenia is unclear. However, it is thought to be an agonist at the trace amine-associated receptor 1 (TAAR1) and serotonin-1A receptor (5-HT1A) receptors.[4] This mechanism of action is unique among available antipsychotics, which generally antagonize dopamine receptors (especially dopamine receptor D2).[5][6]
Pharmacokinetics
The precise pharmacokinetic profile of SEP-363856 has not been reported, though the developer has suggested that the pharmacokinetic data supports once daily dosing.[4]
Research
As of 2018, Sunovion, the maker of another antipsychotic called lurasidone (Latuda), is conducting clinical trials on SEP-363856 in partnership with the preclinical research company PsychoGenics.[2][7][8] The U.S. Food and Drug Administration has granted SEP-363856 the breakthrough therapy designation.[4][9] In addition to schizophrenia, SEP-363856 is also being studied for the treatment of psychosis associated with Parkinson's disease.[9]
References
- "SEP 363856 - AdisInsight". adisinsight.springer.com. Adis International Ltd. Retrieved 29 December 2018.
- Brooks, Megan. "New Psychotropic Drug for Schizophrenia Promising in Early Testing". Medscape. Reuters Health Information. Retrieved 29 December 2018.
- Brooks, Megan. "'Game Changer' for Schizophrenia on the Horizon?". Medscape. WebMD LLC. Retrieved 21 June 2019.
- "Sunovion and PsychoGenics Announce that SEP-363856 Has Received FDA Breakthrough Therapy Designation for the Treatment of People with Schizophrenia". www.bloomberg.com. Bloomberg L.P. Retrieved 21 June 2019.
- Koblan, Kenneth; Hopkins, Seth; Justine, Kent; Hailong, Cheng; Goldman, Robert; Loebel, Antony (2019). "O12.5. Efficacy and Safety of Sep-363856, A Novel Psychotropic Agent with a Non-D2 Mechanism of Action, in the Treatment of Schizophrenia: A 4-Week, Randomized, Placebo-Controlled Trial". Schizophrenia Bulletin. 45: S199. doi:10.1093/schbul/sbz021.269.
- Dedic, Nina; Jones, Philip G.; Hopkins, Seth C.; Lew, Robert; Shao, Liming; Campbell, John E.; Spear, Kerry L.; Large, Thomas H.; Campbell, Una C.; Hanania, Taleen; Leahy, Emer; Koblan, Kenneth S. (2019). "SEP-363856, a Novel Psychotropic Agent with a Unique, Non-D2 Receptor Mechanism of Action". Journal of Pharmacology and Experimental Therapeutics. 371 (1): 1–14. doi:10.1124/jpet.119.260281. PMID 31371483.
- "Sunovion – Our Therapies". www.sunovion.us. Sumitomo Dainippon Pharma Co., Ltd. Retrieved 29 December 2018.
- "About Us". www.psychogenics.com. PsychoGenics. Retrieved 29 December 2018.
- "Drug Receives FDA's Breakthrough Therapy Designation for Treating Individuals with Schizophrenia". Pharmacy Times. Pharmacy & Healthcare Communications, LLC. Retrieved 21 June 2019.