Pyrroloquinoline quinone
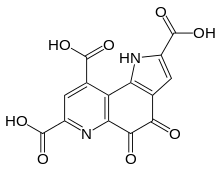
Pyrroloquinoline quinone (PQQ), also called methoxatin, is a redox cofactor. It is found in soil and foods such as kiwifruit, as well as human breast milk.[1] Enzymes containing PQQ are called quinoproteins. Glucose dehydrogenase, one of the quinoproteins, is used as a glucose sensor. PQQ stimulates growth in bacteria.[2]
 | |
| Identifiers | |
|---|---|
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | PQQ+Cofactor |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C14H6N2O8 |
| Molar mass | 330.208 g·mol−1 |
| Density | 1.963 g/cm3 |
| Hazards | |
| Flash point | 569.8 °C (1,057.6 °F; 842.9 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
History
It was discovered by J.G. Hauge as the third redox cofactor after nicotinamide and flavin in bacteria (although he hypothesised that it was naphthoquinone).[3] Anthony and Zatman also found the unknown redox cofactor in alcohol dehydrogenase. In 1979, Salisbury and colleagues[4] as well as Duine and colleagues[5] extracted this prosthetic group from methanol dehydrogenase of methylotrophs and identified its molecular structure. Adachi and colleagues discovered that PQQ was also found in Acetobacter.[6]
Biosynthesis
A novel aspect of PQQ is its biosynthesis in bacteria from a ribosomally translated precursor peptide, PqqA[7]. A glutamic acid and a tyrosine in PqqA are cross-linked by the radical SAM enzyme PqqE in the first step of PqqA modification. Efforts to understand PQQ biosynthesis have contributed to broad interest in radical SAM enzymes and their ability to modify proteins, and an analogous radical SAM enzyme-dependent pathway has since been found that produces the putative electron carrier mycofactocin, using a valine and a tyrosine from the precursor peptide MftA[8].
Controversy regarding role as vitamin
The scientific journal Nature published the 2003 paper by Kasahara and Kato that essentially stated that PQQ was a new vitamin and in 2005, an article by Anthony and Fenton that stated that the 2003 Kasahara and Kato paper drew incorrect and unsubstantiated conclusions.[9] An article in The Proceedings of the National Academy of Sciences in 2018 identified pyrroloquinoline quinone as a “longevity vitamin” not essential for immediate survival, but necessary for long-term health.[10]
References
- Drain, Kelsey (12 February 2017). "Natural Antioxidant Could Prevent Liver Disease". msn.com. Retrieved 14 February 2017.
- Ameyama M, Matsushita K, Shinagawa E, Hayashi M, Adachi O (1988). "Pyrroloquinoline quinone: excretion by methylotrophs and growth stimulation for microorganisms". BioFactors. 1 (1): 51–3. PMID 2855583.
- Hauge JG (1964). "Glucose dehydrogenase of bacterium anitratum: an enzyme with a novel prosthetic group". J Biol Chem. 239: 3630–9. PMID 14257587.
- Salisbury SA, Forrest HS, Cruse WB, Kennard O (1979). "A novel coenzyme from bacterial primary alcohol dehydrogenases". Nature. 280 (5725): 843–4. doi:10.1038/280843a0. PMID 471057.
- Westerling J, Frank J, Duine JA (1979). "The prosthetic group of methanol dehydrogenase from Hyphomicrobium X: electron spin resonance evidence for a quinone structure". Biochem Biophys Res Commun. 87 (3): 719–24. doi:10.1016/0006-291X(79)92018-7. PMID 222269.
- Ameyama M, Matsushita K, Ohno Y, Shinagawa E, Adachi O (1981). "Existence of a novel prosthetic group, PQQ, in membrane-bound, electron transport chain-linked, primary dehydrogenases of oxidative bacteria". FEBS Lett. 130 (2): 179–83. doi:10.1016/0014-5793(81)81114-3. PMID 6793395.
- Goosen N, Huinen RG, van de Putte P (1992). "A 24-amino-acid polypeptide is essential for the biosynthesis of the coenzyme pyrrolo-quinoline-quinone". J Bacteriol. 174 (4): 1426–7. doi:10.1128/jb.174.4.1426-1427.1992. PMC 206443. PMID 1310505.
- Haft DH (2011). "Bioinformatic evidence for a widely distributed, ribosomally produced electron carrier precursor, its maturation proteins, and its nicotinoprotein redox partners". BMC Genomics. 12: 21. doi:10.1186/1471-2164-12-21. PMC 3023750. PMID 21223593.
- Felton LM, Anthony C (2005). "Biochemistry: role of PQQ as a mammalian enzyme cofactor?". Nature. 433 (7025): E10, discussion E11–2. doi:10.1038/nature03322. PMID 15689995.
- Ames, Bruce (15 October 2018). "Prolonging healthy aging: Longevity vitamins and proteins".
- "L-aminoadipate-semialdehyde dehydrogenase (Homo sapiens)". BRENDA. Technische Universität Braunschweig. July 2015. Retrieved 18 July 2015.
- "Pyrroloquinoline quinone (HMDB13636)". Human Metabolome Database. University of Alberta. Retrieved 19 July 2015.
Enzymes containing PQQ are called quinoproteins. PQQ and quinoproteins play a role in the redox metabolism and structural integrity of cells and tissues PMID 2558842. It was reported that aminoadipate semialdehyde dehydrogenase (AASDH) might also use PQQ as a cofactor, suggesting a possibility that PQQ is a vitamin in mammals. PMID 12712191.