Paramecium
Paramecium (also Paramoecium, /ˌpærəˈmiːʃ(i)əm/, PARR-ə-MEE-sh(ee-)əm, /-siəm/, -see-əm)[1] is a genus of unicellular ciliates, commonly studied as a representative of the ciliate group. Paramecia are widespread in freshwater, brackish, and marine environments and are often very abundant in stagnant basins and ponds. Because some species are readily cultivated and easily induced to conjugate and divide, it has been widely used in classrooms and laboratories to study biological processes. Its usefulness as a model organism has caused one ciliate researcher to characterize it as the "white rat" of the phylum Ciliophora.[2]
| Paramecium | |
|---|---|
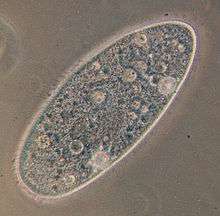 | |
| Paramecium aurelia | |
| Scientific classification | |
| Clade: | SAR |
| Infrakingdom: | Alveolata |
| Phylum: | Ciliophora |
| Class: | Oligohymenophorea |
| Order: | Peniculida |
| Family: | Parameciidae |
| Genus: | Paramecium Müller, 1773 |
| Species | |
|
See text | |
| Synonyms | |
| |
Historical background
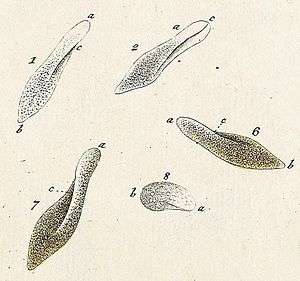
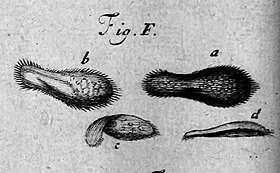
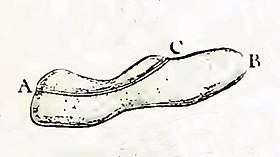
Paramecia were among the first ciliates to be seen by microscopists, in the late 17th century. They were probably known to the Dutch pioneer of protozoology, Antonie van Leeuwenhoek, and were clearly described by his contemporary Christiaan Huygens in a letter of 1678.[3] The earliest known illustration of a Paramecium was published anonymously in Philosophical Transactions of the Royal Society, in 1703.[4]
In 1718, the French mathematics teacher and microscopist Louis Joblot published a description and illustration of a microscopic poisson (fish), which he discovered in an infusion of oak bark in water. Joblot gave this creature the name "Chausson", or "slipper", and the phrase "slipper animalcule" remained in use as a colloquial epithet for Paramecium, throughout the 18th and 19th centuries.[5]
The name "Paramecium" – constructed from the Greek παραμήκης (paramēkēs, "oblong") – was coined in 1752 by the English microscopist John Hill, who applied the name generally to "Animalcules which have no visible limbs or tails, and are of an irregularly oblong figure".[6] In 1773, O. F. Müller, the first researcher to place the genus within the Linnaean system of taxonomy, adopted the name Paramecium, but changed the spelling to Paramœcium. C. G. Ehrenberg, in a major study of the infusoria published in 1838, restored Hill's original spelling for the genus name, and most researchers have followed his lead.[7]
Description
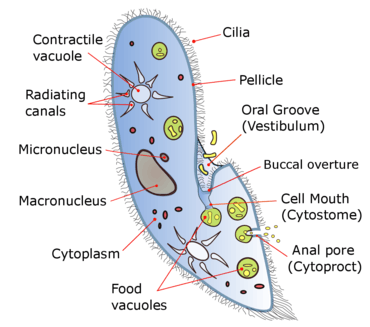
Species of Paramecium range in size from 50 to 330 micrometres (0.0020 to 0.0130 in) in length. Cells are typically ovoid, elongate, foot- or cigar-shaped.
The body of the cell is enclosed by a stiff but elastic structure called the pellicle. This consists of the outer cell membrane (plasma membrane), a layer of flattened membrane-bound sacs called alveoli, and an inner membrane called the epiplasm. The pellicle is not smooth, but textured with hexagonal or rectangular depressions. Each of these polygons is perforated by a central aperture through which a single cilium projects. Between the alveolar sacs of the pellicle, most species of Paramecium have closely spaced spindle-shaped trichocysts, explosive organelles that discharge thin, non-toxic filaments, often used for defensive purposes.[8][9]
Typically, an anal pore (cytoproct) is located on the ventral surface, in the posterior half of the cell. In all species, there is a deep oral groove running from the anterior of the cell to its midpoint. This is lined with inconspicuous cilia which beat continuously, drawing food inside the cell.[10] Paramecia live mainly by heterotrophy, feeding on bacteria and other small organisms. A few species are mixotrophs, deriving some nutrients from endosymbiotic algae (chlorella) carried in the cytoplasm of the cell.[11]
Osmoregulation is carried out by contractile vacuoles, which actively expel water from the cell to compensate for fluid absorbed by osmosis from its surroundings.[12] The number of contractile vacuoles varies from one, to many, depending on species.[10]
Movement
A Paramecium propels itself by whiplash movements of the cilia, which are arranged in tightly spaced rows around the outside of the body. The beat of each cilium has two phases: a fast "effective stroke", during which the cilium is relatively stiff, followed by a slow "recovery stroke", during which the cilium curls loosely to one side and sweeps forward in a counter-clockwise fashion. The densely arrayed cilia move in a coordinated fashion, with waves of activity moving across the "ciliary carpet", creating an effect sometimes likened to that of the wind blowing across a field of grain.[13]
The Paramecium spirals through the water as it progresses. When it happens to encounter an obstacle, the "effective stroke" of its cilia is reversed and the organism swims backward for a brief time, before resuming its forward progress. This is called the avoidance reaction. If it runs into the solid object again, it repeats this process, until it can get past the object.[14]
It has been calculated that a Paramecium expends more than half of its energy in propelling itself through the water.[15] This ciliary method of locomotion has been found to be less than 1% efficient. This low percentage is nevertheless close to the maximum theoretical efficiency that can be achieved by an organism equipped with cilia as short as those of the members of Paramecium.[16]
Gathering food
Paramecia feed on microorganisms like bacteria, algae, and yeasts. To gather food, the Paramecium makes movements with cilia to sweep prey organisms, along with some water, through the oral groove (vestibulum, or vestibule), and into the cell. The food passes from the cilia-lined oral groove into a narrower structure known as the buccal cavity (gullet). From there, food particles pass through a small opening called the cytostome, or cell mouth, and move into the interior of the cell. As food enters the cell, it is gathered into food vacuoles, which are periodically closed off and released into the cytoplasm, where they begin circulating through the cell body. The food vacuoles are circulated by the streaming movement of the cell contents, a process called cyclosis or cytoplasmic streaming. As a food vacuole moves along, enzymes from the cytoplasm enter it, to digest the contents. As enzymatic digestion proceeds, the vacuole contents become more acidic. Within five minutes of a vacuole's formation, pH of its contents drops from 7 to 3.[17] As digested nutrients pass into the cytoplasm, the vacuole shrinks. When the vacuole, with its fully digested contents, reaches the anal pore, it ruptures, expelling its waste contents to the environment, outside the cell.[18][19][20]
Symbiosis
Some species of Paramecium form mutualistic relationships with other organisms. Paramecium bursaria and Paramecium chlorelligerum harbour endosymbiotic green algae, from which they derive nutrients and a degree of protection from predators such as Didinium nasutum.[21][22] Numerous bacterial endosymbionts have been identified in species of Paramecium.[23] Some intracellular bacteria, known as Kappa particles, give Paramecia that have them the ability to kill other strains of Paramecium that lack Kappa.[23]
Genome
The genome of the species Paramecium tetraurelia has been sequenced, providing evidence for three whole-genome duplications.[24]
In some ciliates, like Stylonychia and Paramecium, only UGA is decoded as a stop codon, while UAG and UAA are reassigned as sense codons (that is, when a standard amino acid is 'encoded' by a stop codon), coding for the amino acid glutamic acid.[25]
Learning
The question of whether Paramecia exhibit learning has been the object of a great deal of experimentation, yielding equivocal results. However, a study published in 2006 seems to show that Paramecium caudatum may be trained, through the application of a 6.5 volt electric current, to discriminate between brightness levels.[26] This experiment has been cited as a possible instance of cell memory, or epigenetic learning in organisms with no nervous system.[27] However, another study in 2017 suggested that the Paramecia can only learn to associate the bright side of its swimming medium to electric current and not the dark side.[28] The same study suggested a molecular mechanism for learning in the Paramecia.[29][28]
Reproduction and sexual phenomena
Like all ciliates, Paramecium has a dual nuclear apparatus, consisting of a polyploid macronucleus, and one or more diploid micronuclei. The macronucleus controls non-reproductive cell functions, expressing the genes needed for daily functioning. The micronucleus is the generative, or germline nucleus, containing the genetic material that is passed along from one generation to the next.[30]
Paramecium reproduces asexually, by binary fission. During reproduction, the macronucleus splits by a type of amitosis, and the micronuclei undergo mitosis. The cell then divides transversally, and each new cell obtains a copy of the micronucleus and the macronucleus.[2]
Fission may occur spontaneously, in the course of the vegetative cell cycle. Under certain conditions, it may be preceded by self-fertilization (autogamy),[31] or it may follow conjugation, a sexual phenomenon in which Paramecium of compatible mating types fuse temporarily and exchange genetic material. During conjugation, the micronuclei of each conjugant divide by meiosis and the haploid gametes pass from one cell to the other. The gametes of each organism then fuse to form diploid micronuclei. The old macronuclei are destroyed, and new ones are developed from the new micronuclei.[30]
Autogamy or conjugation can be induced by shortage of food at certain points in the Paramecium life cycle.[32]
Aging
In the asexual fission phase of growth, during which cell divisions occur by mitosis rather than meiosis, clonal aging occurs leading to a gradual loss of vitality. In some species, such as the well studied Paramecium tetraurelia, the asexual line of clonally aging Paramecia loses vitality and expires after about 200 fissions if the cells fail to undergo autogamy or conjugation. The basis for clonal aging was clarified by transplantation experiments of Aufderheide in 1986.[33] When macronuclei of clonally young Paramecia were injected into Paramecia of standard clonal age, the lifespan (clonal fissions) of the recipient was prolonged. In contrast, transfer of cytoplasm from clonally young Paramecia did not prolong the lifespan of the recipient. These experiments indicated that the macronucleus, rather than the cytoplasm, is responsible for clonal aging. Other experiments by Smith-Sonneborn,[34] Holmes and Holmes,[35] and Gilley and Blackburn[36] demonstrated that, during clonal aging, DNA damage increases dramatically.[37] Thus, DNA damage in the macronucleus appears to be the cause of aging in P. tetraurelia. In this single-celled protist, aging appears to proceed as it does in multicellular eukaryotes, as described in DNA damage theory of aging.
Meiosis and rejuvenation
When clonally aged P. tetraurelia are stimulated to undergo meiosis in association with either conjugation or automixis, the genetic descendants are rejuvenated, and are able to have many more mitotic binary fission divisions. During either of these processes, the micronuclei of the cell(s) undergo meiosis, the old macronucleus disintegrates and a new macronucleus is formed by replication of the micronuclear DNA that had recently undergone meiosis. There is apparently little, if any, DNA damage in the new macronucleus. These findings further solidify that clonal aging is due, in large part, to a progressive accumulation of DNA damage; and that rejuvenation is due to the repair of this damage in the micronucleus during meiosis. Meiosis appears to be an adaptation for DNA repair and rejuvenation in P. tetraurelia.
Video gallery
- Paramecium bursaria, a species with symbiotic algae
- Paramecium putrinum
- Paramecium binary fission
- Paramecium in conjugation
- Paramecium caudatum
List of species
Paramecium aurelia species complex:
- Paramecium primaurelia
- Paramecium biaurelia
- Paramecium triaurelia
- Paramecium tetraurelia
- Paramecium pentaurelia
- Paramecium sexaurelia
- Paramecium septaurelia
- Paramecium octaurelia
- Paramecium novaurelia
- Paramecium decaurelia
- Paramecium undecaurelia
- Paramecium dodecaurelia
- Paramecium tredecaurelia
- Paramecium quadecaurelia
- Paramecium sonneborni
Other species:
- Paramecium buetschlii
- Paramecium bursaria
- Paramecium calkinsi
- Paramecium caudatum
- Paramecium chlorelligerum
- Paramecium duboscqui
- Paramecium grohmannae
- Paramecium jenningsi
- Paramecium multimicronucleatum
- Paramecium nephridiatum
- Paramecium polycaryum
- Paramecium putrinum
- Paramecium schewiakoffi
- Paramecium woodruffi
References
- "paramecium". Merriam-Webster Dictionary.
- Lynn, Denis (2008). The Ciliated Protozoa: Characterization, Classification, and Guide to the Literature. Springer Science & Business Media. p. 279. ISBN 9781402082399.
- Dobell, Clifford (1932). Antony van Leeuwenhoek and his "Little Animals" (1960 ed.). New York: Dover. pp. 164–165. ISBN 978-0-486-60594-4.
- Dolan, John R. (2019-08-01). "Unmasking "The Eldest Son of The Father of Protozoology": Charles King". Protist. 170 (4): 374–384. doi:10.1016/j.protis.2019.07.002. ISSN 1434-4610. PMID 31479910.
- Joblot, Louis (1718). Description et usages de Plusieurs Nouveaux Microscopes, tant simple que composez (in French). 2. Paris: Jacques Collombat. p. 79.
- Hill, John (1752). An History of Animals. Paris: Thomas Osborne. p. 5.
- Woodruff, Lorande Loss (September 1921). "The structure, life history, and intrageneric relationships of Paramecium calkinsi, sp. nov". The Biological Bulletin. 41 (3): 171–180. doi:10.2307/1536748. JSTOR 1536748.
- Lynn, Denis (2008). The Ciliated Protozoa: Characterization, Classification, and Guide to the Literature (3 ed.). Springer Netherlands. ISBN 9781402082382.
- Wichterman, R. (2012-12-06). The Biology of Paramecium. Springer Science & Business Media. ISBN 9781475703726.
- Curds, Colin R.; Gates, Michael; Roberts, David McL. (1983). British and other freshwater ciliated protozoa. 2. Cambridge University Press. p. 126.
- Esteban, Genoveva F.; Fenchel, Tom; Finlay, Bland J. (2010). "Mixotrophy in Ciliates". Protist. 161 (5): 621–641. doi:10.1016/j.protis.2010.08.002. PMID 20970377.
- Reece, Jane B. (2011). Campbell Biology. San Francisco: Pearson Education. p. 134. ISBN 9780321558237.
- Blake, John R.; Sleigh, Michael A. (February 1974). "Mechanics of ciliary locomotion". Biological Reviews. 49 (1): 85–125. doi:10.1111/j.1469-185x.1974.tb01299.x. PMID 4206625.
- Ogura, A., and K. Takahashi. "Artificial deciliation causes loss of calcium-dependent responses in Paramecium" (1976): 170–172.
- Katsu-Kimura, Yumiko; et al. (2009). "Substantial energy expenditure for locomotion in ciliates verified by means of simultaneous measurement of oxygen consumption rate and swimming speed". Journal of Experimental Biology. 212 (12): 1819–1824. doi:10.1242/jeb.028894. PMID 19482999.
- Osterman, Natan; Vilfan, Andrej (September 20, 2011). "Finding the ciliary beating pattern with optimal efficiency" (PDF). Proceedings of the National Academy of Sciences. 108 (38): 15727–15732. arXiv:1107.4273. Bibcode:2011PNAS..10815727O. doi:10.1073/pnas.1107889108. PMC 3179098. PMID 21896741.
- Wichterman, R. (1986). The Biology of Paramecium. Springer US. pp. 200–1. ISBN 9781475703740.
- Wichterman, Ralph (1985). The Biology of Paramecium. New York: Plenum Press. pp. 88–90. ISBN 978-1-4757-0374-0.
- Reece, Jane B.; et al. (2011). Campbell Biology. San Francisco: Pearson Education. p. 584. ISBN 9780321558237.
- Mast, S. O. (February 1947). "The food-vacuole in Paramecium". The Biological Bulletin. 92 (1): 31–72. doi:10.2307/1537967. JSTOR 1537967.
- Berger, Jacques (1980). "Feeding Behaviour of Didinium nasutum on Paramecium bursaria with Normal or Apochlorotic Zoochlorellae". Journal of General Microbiology. 118 (2): 397–404. doi:10.1099/00221287-118-2-397.
- Kreutz, Martin; Stoeck, Thorsten; Foissner, Wilhelm (2012). "Morphological and Molecular Characterization of Paramecium (Viridoparamecium nov. subgen.) chlorelligerum Kahl (Ciliophora)". Journal of Eukaryotic Microbiology. 59 (6): 548–563. doi:10.1111/j.1550-7408.2012.00638.x. PMC 3866650. PMID 22827482.
- Preer, John R., Jr.; Preer, Louise B.; Jurand, Artur (June 1974). "Kappa and Other Endosymbionts in Paramecium aurelia". Bacteriological Reviews. 38 (2): 113–163. PMC 413848. PMID 4599970.
- Aury, Jean-Marc; Jaillon, Oliver; Wincker, Patrick; et al. (November 2006). "Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia". Nature. 444 (7116): 171–8. Bibcode:2006Natur.444..171A. doi:10.1038/nature05230. PMID 17086204.
- Lekomtsev, Sergey; Kolosov, Petr; Bidou, Laure; Frolova, Ludmila; Rousset, Jean-Pierre; Kisselev, Lev (June 26, 2007). "Different modes of stop codon restriction by the Stylonychia and Paramecium eRF1 translation termination factors". Proceedings of the National Academy of Sciences of the United States of America. 104 (26): 10824–9. Bibcode:2007PNAS..10410824L. doi:10.1073/pnas.0703887104. PMC 1904165. PMID 17573528.
- Armus, Harvard L.; Montgomery, Amber R.; Jellison, Jenny L. (Fall 2006). "Discrimination Learning in Paramecia (P. caudatum)". The Psychological Record. 56 (4): 489–498. doi:10.1007/BF03396029.
- Ginsburg, Simona; Jablonka, Eva (2009). "Epigenetic learning in non-neural organisms". Journal of Biosciences. 34 (4): 633–646. doi:10.1007/s12038-009-0081-8. PMID 19920348.
- Alipour, Abolfazl; Dorvash, Mohammadreza; Yeganeh, Yasaman; Hatam, Gholamreza (2017-11-27). "Paramecium Learning: New Insights and Modifications". doi:10.1101/225250. Cite journal requires
|journal=(help) - Alipour, Abolfazl; Dorvash, Mohammadreza; Yeganeh, Yasaman; Hatam, Gholamreza; Seradj, Seyed Hasan (2017-05-22). "Possible Molecular Mechanisms for Paramecium Learning". Journal of Advanced Medical Sciences and Applied Technologies. 3 (1): 39. doi:10.18869/nrip.jamsat.3.1.39. ISSN 2423-5903.
- Prescott, D. M.; et al. (1971). "DNA of ciliated protozoa". Chromosoma. 34 (4): 355–366. doi:10.1007/bf00326311.
- Berger, James D. (October 1986). "Autogamy in Paramecium cell cycle stage-specific commitment to meiosis". Experimental Cell Research. 166 (2): 475–485. doi:10.1016/0014-4827(86)90492-1. PMID 3743667.
- Beale, Geoffrey; Preer, John R., Jr. (2008). Paramecium: Genetics and Epigenetics. CRC. ISBN 9780203491904.
- Aufderheide, Karl J. (1986). "Clonal aging in Paramecium tetraurelia. II. Evidence of functional changes in the macronucleus with age". Mechanisms of Ageing and Development. 37 (3): 265–279. doi:10.1016/0047-6374(86)90044-8. PMID 3553762.
- Smith-Sonneborn, J. (1979). "DNA repair and longevity assurance in Paramecium tetraurelia". Science. 203 (4385): 1115–1117. Bibcode:1979Sci...203.1115S. doi:10.1126/science.424739. PMID 424739.
- Holmes, George E.; Holmes, Norreen R. (July 1986). "Accumulation of DNA damages in aging Paramecium tetraurelia". Molecular and General Genetics MGG. 204 (1): 108–114. doi:10.1007/bf00330196. PMID 3091993.
- Gilley, David; Blackburn, Elizabeth H. (1994). "Lack of telomere shortening during senescence in Paramecium" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 91 (5): 1955–1958. Bibcode:1994PNAS...91.1955G. doi:10.1073/pnas.91.5.1955. PMC 43283. PMID 8127914.
- Bernstein, H; Bernstein, C (1991). Aging, Sex, and DNA Repair. San Diego: Academic Press. pp. 153–156. ISBN 978-0120928606.
| Wikispecies has information related to Paramecium |