Oxalic acid
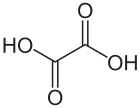
Oxalic acid is an organic compound with the formula C2H2O4. It is a white crystalline solid that forms a colorless solution in water. Its condensed formula is HOOCCOOH, reflecting its classification as the simplest dicarboxylic acid.
 | |||
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Oxalic acid[1] | |||
| Systematic IUPAC name
Ethanedioic acid[1] | |||
| Other names
Wood bleach | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
| 3DMet | |||
Beilstein Reference |
385686 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.123 | ||
| EC Number |
| ||
Gmelin Reference |
2208 | ||
| KEGG | |||
| MeSH | Oxalic+acid | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 3261 | ||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
C2H2O4 | ||
| Molar mass | 90.034 g·mol−1 (anhydrous) 126.065 g·mol−1 (dihydrate) | ||
| Appearance | White crystals | ||
| Odor | odorless | ||
| Density | 1.90 g·cm−3 (anhydrous, at 17 °C)[2] 1.653 g·cm−3 (dihydrate) | ||
| Melting point | 189 to 191 °C (372 to 376 °F; 462 to 464 K) 101.5 °C (214.7 °F; 374.6 K) dihydrate | ||
Solubility in water |
90-100 g/L (20 °C)[2] | ||
| Solubility | 237 g/L (15 °C) in ethanol 14 g/L (15 °C) in diethyl ether [3] | ||
| Vapor pressure | <0.001 mmHg (20 °C)[4] | ||
| Acidity (pKa) | 1.25, 4.14[5] | ||
| Conjugate base | Hydrogenoxalate | ||
Magnetic susceptibility (χ) |
-60.05·10−6 cm3/mol | ||
| Pharmacology | |||
| QP53AG03 (WHO) | |||
| Hazards | |||
| Main hazards | corrosive | ||
| Safety data sheet | External MSDS | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 166 °C (331 °F; 439 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LDLo (lowest published) |
1000 mg/kg (dog, oral) 1400 mg/kg (rat) 7500 mg/kg (rat, oral)[6] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 1 mg/m3[4] | ||
REL (Recommended) |
TWA 1 mg/m3 ST 2 mg/m3[4] | ||
IDLH (Immediate danger) |
500 mg/m3[4] | ||
| Related compounds | |||
Related compounds |
oxalyl chloride disodium oxalate calcium oxalate phenyl oxalate ester | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Its acid strength is much greater than that of acetic acid. Oxalic acid is a reducing agent[7] and its conjugate base, known as oxalate (C
2O2−
4), is a chelating agent for metal cations. Typically, oxalic acid occurs as the dihydrate with the formula C2H2O4·2H2O.
It occurs naturally in many foods, but excessive ingestion of oxalic acid or prolonged skin contact can be dangerous.
Its name comes from the fact that early investigators isolated oxalic acid from wood-sorrel (Oxalis) flowering plants.
History
The preparation of salts of oxalic acid from plants had been known, at the latest, since 1745, when the Dutch botanist and physician Herman Boerhaave isolated a salt from sorrel.[8] By 1773, François Pierre Savary of Fribourg, Switzerland had isolated oxalic acid from its salt in sorrel.[9]
In 1776, Swedish chemists Carl Wilhelm Scheele and Torbern Olof Bergman[10] produced oxalic acid by reacting sugar with concentrated nitric acid; Scheele called the acid that resulted socker-syra or såcker-syra (sugar acid). By 1784, Scheele had shown that "sugar acid" and oxalic acid from natural sources were identical.[11]
In 1824, the German chemist Friedrich Wöhler obtained oxalic acid by reacting cyanogen with ammonia in aqueous solution.[12] This experiment may represent the first synthesis of a natural product.[13]
Preparation
Oxalic acid is mainly manufactured by the oxidation of carbohydrates or glucose using nitric acid or air in the presence of vanadium pentoxide. A variety of precursors can be used including glycolic acid and ethylene glycol.[14] A newer method entails oxidative carbonylation of alcohols to give the diesters of oxalic acid:
- 4 ROH + 4 CO + O2 → 2 (CO2R)2 + 2 H2O
These diesters are subsequently hydrolyzed to oxalic acid. Approximately 120,000 tonnes are produced annually.[13]
Historically oxalic acid was obtained exclusively by using caustics, such as sodium or potassium hydroxide, on sawdust.[15]
Laboratory methods
Although it can be readily purchased, oxalic acid can be prepared in the laboratory by oxidizing sucrose using nitric acid in the presence of a small amount of vanadium pentoxide as a catalyst.[16]
The hydrated solid can be dehydrated with heat or by azeotropic distillation.[17]
Developed in the Netherlands, an electrocatalysis by a copper complex helps reduce carbon dioxide to oxalic acid;[18] this conversion uses carbon dioxide as a feedstock to generate oxalic acid.
Structure
Anhydrous oxalic acid exists as two polymorphs; in one the hydrogen-bonding results in a chain-like structure whereas the hydrogen bonding pattern in the other form defines a sheet-like structure.[19] Because the anhydrous material is both acidic and hydrophilic (water seeking), it is used in esterifications.
Reactions
Oxalic acid is a relatively strong acid, despite being a carboxylic acid:
C2O4H2 ⇌ C2O4H− + H+ pKa = 1.27 C2O4H− ⇌ C
2O2−
4 + H+pKa = 4.27
Oxalic acid undergoes many of the reactions characteristic of other carboxylic acids. It forms esters such as dimethyl oxalate (m.p. 52.5 to 53.5 °C (126.5 to 128.3 °F)).[20] It forms an acid chloride called oxalyl chloride.
Oxalate, the conjugate base of oxalic acid, is an excellent ligand for metal ions, e.g. the drug oxaliplatin.
Oxalic acid and oxalates can be oxidized by permanganate in an autocatalytic reaction.[21]
Oxalic acid's pKa values vary in the literature from 1.25-1.46 and 3.81-4.40.[22][23][24] The 100th ed of the CRC, released in 2019 has values of 1.25 and 3.81.[25]
Occurrence
Biosynthesis
At least two pathways exist for the enzyme-mediated formation of oxalate. In one pathway, oxaloacetate, a component of the Krebs citric acid cycle, is hydrolyzed to oxalate and acetic acid by the enzyme oxaloacetase:[26]
- [O2CC(O)CH2CO2]2− + H2O → C
2O2−
4 + CH
3CO−
2 + H+
It also arises from the dehydrogenation of glycolic acid, which is produced by the metabolism of ethylene glycol.
Occurrence in foods and plants
Calcium oxalate is the most common component of kidney stones. Early investigators isolated oxalic acid from wood-sorrel (Oxalis). Members of the spinach family and the brassicas (cabbage, broccoli, brussels sprouts) are high in oxalates, as are sorrel and umbellifers like parsley.[27] Rhubarb leaves contain about 0.5% oxalic acid, and jack-in-the-pulpit (Arisaema triphyllum) contains calcium oxalate crystals. Similarly, the Virginia creeper, a common decorative vine, produces oxalic acid in its berries as well as oxalate crystals in the sap, in the form of raphides. Bacteria produce oxalates from oxidation of carbohydrates.[13]
Plants of the genus Fenestraria produce optical fibers made from crystalline oxalic acid to transmit light to subterranean photosynthetic sites.[28]
Carambola, also known as starfruit, also contains oxalic acid along with caramboxin.
The formation of naturally occurring calcium oxalate patinas on certain limestone and marble statues and monuments has been proposed to be caused by the chemical reaction of the carbonate stone with oxalic acid secreted by lichen or other microorganisms.[29][30]
Other
Oxidized bitumen or bitumen exposed to gamma rays also contains oxalic acid among its degradation products. Oxalic acid may increase the leaching of radionuclides conditioned in bitumen for radioactive waste disposal.[31]
Biochemistry
The conjugate base of oxalic acid is the hydrogenoxalate anion, and its conjugate base (oxalate) is a competitive inhibitor of the lactate dehydrogenase (LDH) enzyme.[32] LDH catalyses the conversion of pyruvate to lactic acid (end product of the fermentation (anaerobic) process) oxidising the coenzyme NADH to NAD+ and H+ concurrently. Restoring NAD+ levels is essential to the continuation of anaerobic energy metabolism through glycolysis. As cancer cells preferentially use anaerobic metabolism (see Warburg effect) inhibition of LDH has been shown to inhibit tumor formation and growth,[33] thus is an interesting potential course of cancer treatment.
Applications
About 25% of produced oxalic acid will be used as a mordant in dyeing processes. It is used in bleaches, especially for pulpwood. It is also used in baking powder[13] and as a third reagent in silica analysis instruments.
Cleaning
Oxalic acid's main applications include cleaning or bleaching, especially for the removal of rust (iron complexing agent). Its utility in rust removal agents is due to its forming a stable, water-soluble salt with ferric iron, ferrioxalate ion.
Extractive metallurgy
Oxalic acid is an important reagent in lanthanide chemistry. Hydrated lanthanide oxalates form readily in very strongly acidic solutions in a densely crystalline, easily filtered form, largely free of contamination by nonlanthanide elements. Thermal decomposition of these oxalate gives the oxides, which is the most commonly marketed form of these elements.
Niche uses
Oxalic acid is used by some beekeepers as a miticide against the parasitic varroa mite.[34]
Oxalic acid is used to clean minerals.[35][36]
Oxalic acid is sometimes used in the aluminum anodizing process, with or without sulfuric acid. Compared to sulfuric acid anodizing, the coatings obtained are thinner and exhibit lower surface roughness.
Content in food items
| Vegetable | Oxalic acid (g/100 g)a |
|---|---|
| Amaranth | 1.09 |
| Asparagus | 0.13 |
| Beans, snap | 0.36 |
| Beet leaves | 0.61 |
| Beetroot | 0.06[38] |
| Broccoli | 0.19 |
| Brussels sprouts | 0.02[38] |
| Cabbage | 0.10 |
| Carrot | 0.50 |
| Cassava | 1.26 |
| Cauliflower | 0.15 |
| Celery | 0.19 |
| Chicory | 0.2 |
| Chives | 1.48 |
| Collards | 0.45 |
| Coriander | 0.01 |
| Corn, sweet | 0.01 |
| Cucumber | 0.02 |
| Eggplant | 0.19 |
| Endive | 0.11 |
| Garlic | 0.05 |
| Kale | 0.02 |
| Lettuce | 0.33 |
| Okra | 0.05 |
| Onion | 0.05 |
| Parsley | 0.04 |
| Parsnip | 0.04 |
| Pea | 0.05 |
| Bell pepper | 0.04 |
| Potato | 0.05 |
| Purslane | 1.31 |
| Radish | 0.48 |
| Rhubarb leaves | 0.52[39] |
| Rutabaga | 0.03 |
| Spinach | 0.97 (ranges from .65 to 1.3 grams per 100 grams on fresh weight basis)[40] |
| Squash | 0.02 |
| Sweet potato | 0.24 |
| Swiss Chard, green | 0.96 [38] |
| Tomato | 0.05 |
| Turnip | 0.21 |
| Turnip greens | 0.05 |
| Watercress | 0.31 |
Toxicity
Oxalic acid in concentrated form can have harmful effects through contact and if ingested; manufacturers provide details in Material Safety Data Sheets (MSDS). It is not identified as mutagenic or carcinogenic, although there is a study suggesting it might cause breast cancer;[41] there is a possible risk of congenital malformation in the fetus; may be harmful if inhaled, and is extremely destructive to tissue of mucous membranes and upper respiratory tract; harmful if swallowed; harmful to and destructive of tissue and causes burns if absorbed through the skin or is in contact with the eyes. Symptoms and effects include a burning sensation, cough, wheezing, laryngitis, shortness of breath, spasm, inflammation and edema of the larynx, inflammation and edema of the bronchi, pneumonitis, pulmonary edema.[42]
In humans, ingested oxalic acid has an oral LDLo (lowest published lethal dose) of 600 mg/kg.[43] It has been reported that the lethal oral dose is 15 to 30 grams.[44]
Oxalate may enter cells where it is known to cause mitochondrial dysfunction.[45]
The toxicity of oxalic acid is due to kidney failure caused by precipitation of solid calcium oxalate,[46] the main component of calcium kidney stones. Oxalic acid can also cause joint pain due to the formation of similar precipitates in the joints. Ingestion of ethylene glycol results in oxalic acid as a metabolite which can also cause acute kidney failure.
Notes
^a Unless otherwise cited, all measurements are based on raw vegetable weights with original moisture content.
References
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. P001–P004. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- Radiant Agro Chem. "Oxalic Acid MSDS".
- NIOSH Pocket Guide to Chemical Hazards. "#0474". National Institute for Occupational Safety and Health (NIOSH).
- Bjerrum, J., et al. (1958) Stability Constants, Chemical Society, London.
- "Oxalic acid". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Ullmann's Encyclopedia of Industrial Chemistry. Wiley. 2005. pp. 17624/28029. doi:10.1002/14356007. ISBN 9783527306732.
- See:
- Herman Boerhaave, Elementa Chemiae (Basil, Switzerland: Johann Rudolph Im-hoff, 1745), volume 2, pp. 35-38. (in Latin) From p. 35: "Processus VII. Sal nativum plantarum paratus de succo illarum recens presso. Hic Acetosae." (Procedure 7. A natural salt of plants prepared from their freshly pressed juice. This [salt obtained] from sorrel.)
- Henry Enfield Roscoe and Carl Schorlemmer, ed.s, A Treatise on Chemistry (New York, New York: D. Appleton and Co., 1890), volume 3, part 2, p. 105.
- See also Wikipedia's articles "Oxalis acetosella" and "Potassium hydrogen oxalate".
- See:
- François Pierre Savary, Dissertatio Inauguralis De Sale Essentiali Acetosellæ [Inaugural dissertation on the essential salt of wood sorrel] (Jean François Le Roux, 1773). (in Latin) Savary noticed that when he distilled sorrel salt (potassium hydrogen oxalate), crystals would sublimate onto the receiver. From p. 17: "Unum adhuc circa liquorem acidum, quem sal acetosellae tam sincerissimum a nobis paratum quam venale destillatione fundit phoenomenon erit notandum, nimirum quod aliquid ejus sub forma sicca crystallina lateribus excipuli accrescat, ..." (One more [thing] will be noted regarding the acid liquid, which furnished for us sorrel salt as pure as commercial distillations, [it] produces a phenomenon, that evidently something in dry, crystalline form grows on the sides of the receiver, ...) These were crystals of oxalic acid.
- Leopold Gmelin with Henry Watts, trans., Hand-book of Chemistry (London, England: Cavendish Society, 1855), volume 9, p. 111.
- See:
- Torbern Bergman with Johan Afzelius (1776) Dissertatio chemica de acido sacchari [Chemical dissertation on sugar acid] (Uppsala, Sweden: Edman, 1776).
- Torbern Bergman, Opuscula Physica et Chemica, (Leipzig (Lipsia), (Germany): I.G. Müller, 1776), volume 1, "VIII. De acido Sacchari," pp. 238-263.
- Carl Wilhelm Scheele (1784) "Om Rhabarber-jordens bestånds-delar, samt sått at tilreda Acetosell-syran" (On rhubarb-earth's constituents, as well as ways of preparing sorrel-acid), Kungliga Vetenskapsakademiens Nya Handlingar [New Proceedings of the Royal Academy of Science], 2nd series, 5 : 183-187. (in Swedish) From p. 187: "Således finnes just samma syra som vi genom konst af socker med tilhjelp af salpeter-syra tilreda, redan förut af naturen tilredd uti o̊rten Acetosella." (Thus it is concluded [that] the very same acid as we prepare artificially by means of sugar with the help of nitric acid, [was] previously prepared naturally in the herb acetosella [i.e., sorrel].)
- See:
- F. Wöhler (1824) "Om några föreningar af Cyan" (On some compounds of cyanide), Kungliga Vetenskapsakademiens Handlingar [Proceedings of the Royal Academy of Science], pp. 328-333. (in Swedish)
- Reprinted in German as: F. Wöhler (1825) "Ueber Cyan-Verbindungen" (On cyanide compounds), Annalen der Physik und Chemie, 2nd series, 3 : 177-182.
- Wilhelm Riemenschneider, Minoru Tanifuji "Oxalic acid" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a18_247.
- Eiichi, Yonemitsu; Tomiya, Isshiki; Tsuyoshi, Suzuki and Yukio, Yashima "Process for the production of oxalic acid", U.S. Patent 3,678,107, priority date March 15, 1969
- Von Wagner, Rudolf (1897). Manual of chemical technology. New York: D. Appleton & Co. p. 499.
- Practical Organic Chemistry by Julius B. Cohen, 1930 ed. preparation #42
- Clarke H. T.;. Davis, A. W. (1941). "Oxalic acid (anhydrous)". Organic Syntheses: 421.CS1 maint: multiple names: authors list (link); Collective Volume, 1
- Bouwman, Elisabeth; Angamuthu, Raja; Byers, Philip; Lutz, Martin; Spek, Anthony L. (July 15, 2010). "Electrocatalytic CO2 Conversion to Oxalate by a Copper Complex". Science. 327 (5393): 313–315. Bibcode:2010Sci...327..313A. CiteSeerX 10.1.1.1009.2076. doi:10.1126/science.1177981. PMID 20075248.
- Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- Bowden, E. (1943). "Methyl oxalate". Organic Syntheses: 414.; Collective Volume, 2
- Kovacs K.A.; Grof P.; Burai L.; Riedel M. (2004). "Revising the mechanism of the permanganate/oxalate reaction". J. Phys. Chem. A. 108 (50): 11026–11031. Bibcode:2004JPCA..10811026K. doi:10.1021/jp047061u.
- Bjerrum, J., et al. (1958) Stability Constants, Chemical Society, London.
- Haynes, W. M. (Ed.). (2014). CRC Handbook of Chemistry and Physics, 95th Edition (95 edition). Boca Raton; London; New York: CRC Press.]
- Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 4936
- Rumble, J. (Ed.). (2019). CRC Handbook of Chemistry and Physics, 100th Edition (100 edition). CRC Press.
- Dutton, M. V.; Evans, C. S. (1996). "Oxalate production by fungi: Its role in pathogenicity and ecology in the soil environment". Canadian Journal of Microbiology. 42 (9): 881–895. doi:10.1139/m96-114..
- Rombauer, Rombauer Becker, and Becker (1931/1997). Joy of Cooking, p.415. ISBN 0-684-81870-1.
- Attenborough, David. "Surviving." The Private Life of Plants: A Natural History of Plant Behaviour. Princeton, NJ: Princeton UP, 1995. 265+. "OpenLibrary.org: The Private Life of Plants" Print.
- Sabbioni, Cristina; Zappia, Giuseppe (2016). "Oxalate patinas on ancient monuments: The biological hypothesis". Aerobiologia. 7: 31–37. doi:10.1007/BF02450015.
- . doi:10.1007/978-3-642-27682-8_27-1 (inactive 2019-08-23). Missing or empty
|title=(help) - EPJ Web of Conferences
- Novoa, William; Alfred Winer; Andrew Glaid; George Schwert (1958). "Lactic Dehydrogenase V. inhibition by Oxamate and Oxalate". Journal of Biological Chemistry. 234 (5): 1143–8. PMID 13654335.
- Le, Anne; Charles Cooper; Arvin Gouw; Ramani Dinavahi; Anirban Maitra; Lorraine Deck; Robert Royer; David Vander Jagt; Gregg Semenza; Chi Dang (14 December 2009). "Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression". Proceedings of the National Academy of Sciences. 107 (5): 2037–2042. doi:10.1073/pnas.0914433107. PMC 2836706. PMID 20133848.
- Exploring New Methods for Varroa Mite Control, Yu-Lun Lisa Fu
- Jackson, Faith. "Quartz Crystal Cleaning". bluemooncrystals.com
- "Rock Currier – Cleaning Quartz". mindat.org
- All data not specifically annotated is from Agriculture Handbook No. 8-11, Vegetables and Vegetable Products, 1984. ("Nutrient Data : Oxalic Acid Content of Selected Vegetables". ars.usda.gov)
- Chai, Weiwen; Liebman, Michael (2005). "Effect of Different Cooking Methods on Vegetable Oxalate Content". Journal of Agricultural and Food Chemistry. 53 (8): 3027–30. doi:10.1021/jf048128d. PMID 15826055.
- Pucher, GW; Wakeman, AJ; Vickery, HB (1938). "The organic acids of rhubarb (Rheum hybridium). III. The behavior of the organic acids during culture of excised leaves". Journal of Biological Chemistry. 126 (1): 43.
- Durham, Sharon. "Making Spinach with Low Oxalate Levels". AgResearch Magazine (January 2017). United States Department of Agriculture. Retrieved 26 June 2017.
The scientists analyzed oxalate concentrations in 310 spinach varieties—300 USDA germplasm accessions and 10 commercial cultivars. “These spinach varieties and cultivars displayed oxalate concentrations from 647.2 to 1286.9 mg/100 g on a fresh weight basis,” says Mou.
- Castellaro, Andrés M.; Tonda, Alfredo; Cejas, Hugo H.; Ferreyra, Héctor; Caputto, Beatriz L.; Pucci, Oscar A.; Gil, German A. (2015-10-22). "Oxalate induces breast cancer". BMC Cancer. 15: 761. doi:10.1186/s12885-015-1747-2. ISSN 1471-2407. PMC 4618885. PMID 26493452.
- Oxalic acid dihydrate. MSDS. sigmaaldrich.com
- "Oxalic Acid Material Safety Data Sheet" (PDF). Radiant Indus Chem. Retrieved 2014-05-20.
- "CDC – Immediately Dangerous to Life or Health Concentrations (IDLH): Oxalic acid – NIOSH Publications and Products". cdc.gov
- Patel, Mikita; Yarlagadda, Vidhush; Adedoyin, Oreoluwa; Saini, Vikram; Assimos, Dean G.; Holmes, Ross P.; Mitchell, Tanecia (May 2018). "Oxalate induces mitochondrial dysfunction and disrupts redox homeostasis in a human monocyte derived cell line". Redox Biology. 15: 207–215. doi:10.1016/j.redox.2017.12.003. PMC 5975227. PMID 29272854.
- EMEA Committee for veterinary medicinal products, oxalic acid summary report, December 2003
External links
| Wikimedia Commons has media related to Oxalic acid. |
- Oxalic acid MS Spectrum
- International Chemical Safety Card 0529
- NIOSH Guide to Chemical Hazards (CDC)
- Table: Oxalic acid content of selected vegetables (USDA)
- Alternative link: Table: Oxalic Acid Content of Selected Vegetables (USDA)
- About rhubarb poisoning (The Rhubarb Compendium)
- Oxalosis & Hyperoxaluria Foundation (OHF) The Oxalate Content of Food 2008 (PDF)
- Oxalosis & Hyperoxaluria Foundation (OHF) Diet Information
- Calculator: Water and solute activities in aqueous oxalic acid