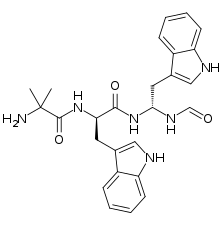
Macimorelin
Macimorelin (INN), or Macrilen (trade name) is a drug being developed by Æterna Zentaris for use in the diagnosis of adult growth hormone deficiency. Macimorelin acetate, the salt formulation, is a synthetic growth hormone secretagogue receptor agonist.[1] Macimorelin acetate is described chemically as D-Tryptophanamide, 2-methylalanyl-N-[(1R)-1-(formylamino)-2-(1H-indol-3-yl)ethyl]-acetate.
 | |
| Names | |
|---|---|
| IUPAC name
2-Amino-N-[(2R)-1-[[(1R)-1-formamido-2-(1H-indol-3-yl)ethyl]amino]-3-1H-indol-3-yl)-1-oxopropan-2-yl]-2-methylpropanamide | |
| Other names
Aib-Trp-gTrp-CHO; AEZS-130; JMV 1843; Macimorelin acetate | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C26H30N6O3 |
| Molar mass | 474.565 g·mol−1 |
| Pharmacology | |
| V04CD06 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
As of January 2014, it was in Phase III clinical trials.[2] The phase III trial for growth hormone deficiency is expected to be complete in December 2016.[3]
As of December 2017, it became FDA-approved as a method to diagnose growth hormone deficiency.[4] Traditionally, growth hormone deficiency was diagnosed via means of insulin tolerance test (IST) or glucagon stimulation test (GST). These two means are done parenterally, whereas Macrilen boasts an oral formulation for ease of administration for patients and providers.
Macimorelin is a growth hormone secretagogue receptor (ghrelin receptor) agonist causing release of growth hormone from the pituitary gland.[5][6][7]
See also
References
- "Macrilen Prescribing Information" (PDF). Retrieved 2018-07-25.
- "Aeterna Zentaris NDA for Macimorelin Acetate in AGHD Accepted for Filing by the FDA". Wall Street Journal. January 6, 2014.
- https://clinicaltrials.gov/ct2/show/NCT02558829
- Research, Center for Drug Evaluation and. "Drug Approvals and Databases - Drug Trials Snapshots: Marcrilen". www.fda.gov. Retrieved 2018-07-25.
- "Macimorelin". NCI Drug Dictionary. National Cancer Institute.
- Koch, Linda (2013). "Growth hormone in health and disease: Novel ghrelin mimetic is safe and effective as a GH stimulation test". Nature Reviews Endocrinology. 9 (6): 315. doi:10.1038/nrendo.2013.89.
- Garcia, J. M.; Swerdloff, R.; Wang, C.; Kyle, M.; Kipnes, M.; Biller, B. M. K.; Cook, D.; Yuen, K. C. J.; Bonert, V.; Dobs, A.; Molitch, M. E.; Merriam, G. R. (2013). "Macimorelin (AEZS-130)-Stimulated Growth Hormone (GH) Test: Validation of a Novel Oral Stimulation Test for the Diagnosis of Adult GH Deficiency". Journal of Clinical Endocrinology & Metabolism. 98 (6): 2422. doi:10.1210/jc.2013-1157. PMC 4207947.