Deferiprone
Deferiprone (tradenames include Ferriprox) is a drug that chelates iron and is used to treat iron overload in thalassaemia major.[1] It was first approved for use in treating thalassaemia major in 1994[2] and had been licensed for use in Europe and Asia for many years while awaiting approval in Canada and the United States.[1] On October 14, 2011, it was approved for use in the US under the FDA's accelerated approval program.[3]
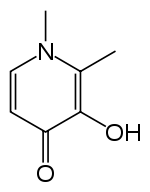 | |
| Clinical data | |
|---|---|
| Trade names | Ferriprox |
| AHFS/Drugs.com | International Drug Names |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Glucuronidation |
| Elimination half-life | 2 to 3 hours |
| Excretion | Renal (75 to 90% in 24 hours) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.157.470 |
| Chemical and physical data | |
| Formula | C7H9NO2 |
| Molar mass | 139.152 g/mol g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Controversy
Deferiprone was at the center of a protracted struggle between Nancy Olivieri, a Canadian haematologist and researcher, and the Hospital for Sick Children and the pharmaceutical company Apotex, that started in 1996 and delayed approval of the drug in North America.[4] Olivieri's data suggested deferiprone leads to progressive hepatic fibrosis.[5][6][7]
See also
References
- Savulescu, J (2004). "Thalassaemia major: The murky story of deferiprone". BMJ. 328 (7436): 358–9. doi:10.1136/bmj.328.7436.358. PMC 341373. PMID 14962851.
- Staff, Cipla. Cipla's History Archived 2015-10-27 at the Wayback Machine
- FDA NEWS RELEASE: FDA Approves Ferripox (deferiprone) to Treat Patients with Excess Iron in the Body, Oct. 14, 2011 https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm275814.htm
- Viens, A M; Savulescu, J (2004). "Introduction to the Olivieri symposium". Journal of Medical Ethics. 30 (1): 1–7. doi:10.1136/jme.2003.006577. PMC 1757126. PMID 14872065.
- Brittenham, G. M; Nathan, D. G; Olivieri, N. F; Porter, J. B; Pippard, M; Vichinsky, E. P; Weatherall, D. J (2003). "Deferiprone and hepatic fibrosis". Blood. 101 (12): 5089–90, author reply 5090–1. doi:10.1182/blood-2002-10-3173. PMID 12788794.
- Wanless, I. R; Sweeney, G; Dhillon, A. P; Guido, M; Piga, A; Galanello, R; Gamberini, M. R; Schwartz, E; Cohen, A. R (2002). "Lack of progressive hepatic fibrosis during long-term therapy with deferiprone in subjects with transfusion-dependent beta-thalassemia". Blood. 100 (5): 1566–9. doi:10.1182/blood-2002-01-0306. PMID 12176871.
- Cribb, Robert (2019-02-27). "UHN patients given unlicensed drug that led to diabetes, liver dysfunction and one death, study finds". The Star. Toronto. Retrieved 2019-02-27.