Antibiotic use in livestock
Antibiotic use in livestock is the use of antibiotics for any purpose in the husbandry of livestock, which includes treatment when ill (therapeutic), treatment of a herd of animals when at least one is diagnosed as ill (metaphylaxis), and preventative treatment (prophylaxis). The use of subtherapeutic doses in animal feed and water[1] to promote growth and improve feed efficiency became illegal in the United States on 1 January 2017, through legislative change enacted by the Food and Drug Administration (FDA), which sought voluntary compliance from drug manufacturers to re-label their antibiotics.[2][3] The use of antibiotics in livestock including poultry may have impacts on human and environmental health. Legislation on antibiotic use in farm animals is being introduced across the globe.
History

In 1910 in the United States, a meat shortage resulted in protests and boycotts.[4][5] After this and other shortages, the public demanded government research into stabilization of food supplies.[4] Since the 1900s, livestock production on United States farms has had to rear larger quantities of animals over a short period of time to meet new consumer demands. It was discovered in the 1940s that feeding subtherapeutic levels of antibiotics improved feed efficiency and accelerated animal growth.[6] Following this discovery, American Cyanamid published research establishing the practice of using antibiotic growth promoters.[4]
By 2001, this practice had grown so much that a report by the Union of Concerned Scientists found that nearly 90% of the total use of antimicrobials in the United States was for non-therapeutic purposes in agricultural production.[7]
Growth stimulation
Certain antibiotics, when given in low, subtherapeutic doses, are known to improve feed conversion efficiency (more output, such as muscle or milk, for a given amount of feed) and may promote greater growth, most likely by affecting gut flora.[8] The drugs listed below can be used to increase feed conversion ratio and weight gain, but are not legally allowed to be used for such purposes any longer in the United States. Some drugs listed below are ionophores, which are not antibiotics and do not pose any potential risk to human health.
| Antibiotic Growth Promoters used in Livestock Production | ||||
|---|---|---|---|---|
| Drug | Class | Livestock | ||
| Bacitracin | Peptide | Beef cattle, chickens, swine, and turkeys; promotes egg production in chickens[9][10] | ||
| Bambermycin | Beef cattle, chickens, swine, and turkeys.[9][10] | |||
| Carbadox | Swine[9] | |||
| Laidlomycin | Beef cattle[9] | |||
| Lasalocid | Ionophore | Beef cattle[9][10] | ||
| Lincomycin | Chickens and swine[9] | |||
| Monensin | Ionophore | Beef cattle and sheep; promotes milk production in dairy cows[9][10] | ||
| Neomycin/ Oxytetracycline | Beef cattle, chickens, swine, and turkeys[9] | |||
| Penicillin | Chickens, swine, and turkeys[9] | |||
| Roxarsone | Chickens and turkeys[9] | |||
| Salinomycin | Ionophore | |||
| Tylosin | Chickens and swine[9] | |||
| Virginiamycin | Peptide | Beef cattle, chickens, swine, turkeys[9][10] | ||
Concentrated animal feeding operations
Concentrated animal feeding operations (CAFO) are large-scale, feed and housing efficient industrial facilities that raise animals in high-densities for the production of low-cost meat, eggs or milk. Most CAFOs produce only one kind of animal to improve efficiency and are classified by the type and number of animals contained, as well as how they discharge their waste water.[11]
The classification by water discharge was implemented in 1972 when CAFOs and their smaller counterpart, AFOs, (animal feeding operations) were identified as potential sources of pollution by the Clean Water Act due to the presence of antibiotics, pathogens, and chemicals in the manure produced. The National Pollutant Discharge Elimination System (NPDES) program sets guidelines for and regulates CAFOs and AFOs.
New regulations added in 2008 under the NPDES portions of the Environmental Protection Agency (EPA) Guidelines regarding local zoning ordinances, health regulations, and Nuisance laws have still not been enforced effectively in these mass animal manufacturing operations.[12]
Manure
The large amounts of manure produced in cattle livestock populations are a problem for many CAFOs. Dependent on size of the operation, there can be 2,800 to 1.6 million tons of manure produced per year.[13] Hog farms in North Carolina produce approximately 10 billion gallons of manure annually.[14] Annually, the livestock population in the United States produces 3 to 20 times more manure than people. However, many operations lack ultimate sewage treatment.[15]
(NRCS_Photo_Gallery).jpg)
The manure produced from concentrated populations of animals can be diseased and have negatives impact on the environment itself. Livestock manure can be tainted by blood, pathogens such as E. coli, antibiotics, growth hormones, chemical additives, etc. Some of this manure can be treated and used as fertilizer by liquefying and spraying it, but larger operations often revert to storing it until it can be disposed of properly.[15] Large hog farms, for example, store animal waste in lagoons on site.[14] Manure is also trucked off site, stored in containers, or held in holding ponds. There can be problems associated with storing manure;[15] manure can have detrimental effects on the surrounding area due to leaking containers or holding ponds. This, known as manure leaching, can lead to manure runoff affecting the ground or soil water by percolation or direct contamination.[16]
Groundwater contamination
When manure runoff or percolation enters a water system, the infecting agents thrive in that environment.[16] Previous studies found that a private well in Idaho contained high levels of veterinary antibiotics as well as additive chemicals. The areas surrounding concentrated animal feeding operations are at particular risk for groundwater or soil water sources of contamination.[13] When manure enters a water source, either underground or above ground, the pathogens and agents that inhabit the manure enters the water as well. Pathogens can survive longer in groundwater than surface water due to lower temperatures and protection from the sun and other harsher elements. Additionally, this water will not be treated until far later in the process, allowing bacterial colonies to grow.[15]
The contamination of groundwater is a major concern and source of disease outbreak in humans, since it is one of the largest sources of water that humans are supplied from.[13] As of 2015, nearly one-third of people in the United States relied on groundwater as their primary source of drinking water.[17] Groundwater also gradually leads to surface waters, such as rivers and streams, spreading the pollution further.[15]
Antibiotic usage

Prior to 2017, 80% of all antibiotics were given as feed additives in the United States.[18] While this is no longer legal, the classic administration of antibiotics in livestock is characterized by a general treatment approach where an entire herd of animals is given the same antibiotic of the same dosage, instead of treating each animal independently based on their symptoms and body size. This can lead to antibiotics not being fully synthesized by the animal and instead being excreted into the urine or manure.[19] This has been tested by levels of antibiotics still present in the manure of these animals.
Antibiotics are also used in livestock to treat individual animals when sick, herds of animals when a few become diseased, and to prevent disease in animals that are at risk. The Centers for Disease Control and Prevention supports the responsible use of antibiotics in food animals, and the efforts the FDA and USDA are putting forth to improve antibiotic use.[20]
Vectors
A vector, in this context, is an organism that transmits disease to another organism. Insects such as flies and mosquitoes have high amounts of breeding grounds and nests of eggs around manure waste, allowing rapid reproduction and fresh vectors for potential disease. Typically with dense populations of livestock, transmission of disease from one animal to another can be on account of insects, such as flies, mosquitoes or ticks, spreading blood from one animal to another.[21] This can be particularly dangerous for sick animals spreading diseases to healthier animals, promoting general malaise in a concentrated area. Additionally, the animals can be infected from other animals' manure making contact with their food; fecal-oral transmission are one of the largest sources for pathogen transmission.[15] Within concentrated animal farming operations, there is no mandatory testing of novel viruses, only reporting known illnesses to the World Organization for Animal Health. Thus, certain mutations or recombinant bacteria strains, which are more efficient in translation to human to human events, can be unnoticed.
Additionally, insect beds around manure pools or containers are a particular threat for contamination. These insects feed and reproduce in the runoff of treated manure, so they can acquire resistant strains of bacteria from blood and the manure of livestock treated with antibiotics. Since most manure holding ponds are on or near the sites of the operations, the insects are not far from livestock populations.[15] These insects are also particularly dangerous because they can spread bacteria or other pathogens to humans by infecting human food. This is observed when treated manure is used as fertilizers or liquefied for spraying and as a result of the unsanitary handling of meat in kitchens.[16]
Use and regulation by country
The use of drugs in food animals is regulated in nearly all countries. Historically, this has been to prevent alteration or contamination of meat, milk, eggs and other products with toxins that are harmful to humans. Treating a sick animal with drugs may lead to some of those drugs remaining in the animal when it is slaughtered or milked. Scientific experiments provide data that shows how long a drug is present in the body of an animal and what the animal's body does to the drug. Of particular concern are drugs that may be passed into milk or eggs. By the use of "drug withdrawal periods: before slaughter or the use of milk or eggs from treated animals, veterinarians and animal owners ensure that the meat, milk and eggs is free of contamination.
Brazil
Brazil is the world's largest exporter of beef. The government regulates antibiotic use in the cattle production industry.[22]
Canada
Because of concerns about antibiotics residues getting into the milk or meat of cattle, the Canadian Food Inspection Agency (CFIA) enforces standards which protect consumers by ensuring that foods produced will not contain antibiotics at a level which will cause harm to consumers. In Canada the veterinary drug regulation consists of two federal government agencies, namely Health Canada and the CFIA, which are responsible for implementing and enforcing the Food and Drugs Act. Testing samples for drug residues include three methods: monitoring, surveillance, and compliance. There are Swab Test On Premises (STOP) procedures to detect antibiotic residues in kidney tissues.[23]
China
China produces and consumes the most antibiotics of all countries.[24] Antibiotic use has been measured by checking the water near factory farms in China[25][26] as well as through animal feces.[27] It was calculated that 38.5 million kg (or 84.9 million lbs) of antibiotics were used in China's swine and poultry production in 2012.[28] The abuse of antibiotics caused severe pollution of soil and surface water in Northern China.[29]
In 2012, U.S. News & World Report described the Chinese government's regulation of antibiotics in livestock production as "weak".[30]
On the UK 5 Year Antimicrobial Resistance(AMR) Strategy 2013-2018, the importance of addressing AMR negative effects on animal health has been considered as same as human health. Serval scientific partnerships with low-middle income countries would be established.[31] UK-China Newton fund has started to build multi-discipline collaboration cross the border to stop the increasing global burden caused by AMR.[32] To achieve the goal of citizen public health and food safety, “The National action Plan on Controlling Antibiotic-Resistance Bacteria on animal origins(2016-2020)” has been published by Ministry of Agriculture and Rural Affairs of People's Republic of China since 2017. This plan is fully integrated with the concept of one health. It covers not only the research and development, but also social context.
The following aims should be achieved by 2020:[33][34]
- Implementation of Exit Plan, to encourage the drop of antibiotics as the growth promoters
- Regulation of drug market, to strengthen the registration and management of veterinary antibiotics
- Improvements on AMR surveillance system
- Strengthening on the testing of antibacterial residue
- Exemplification on effective models of decreasing the use on antibiotics
- Education on public and professions
European Union
In 1999, the European Union (EU) implemented an antibiotic resistance monitoring program and a plan to phase out antibiotic use for the purposes of growth promotion by 2006.[35] The European Union banned the use of antibiotics as growth agents starting on 1 January 2006 with Regulation (EC) No 1831/2003.[36] In Germany, 1,734 tons of antimicrobial agents were used for animals in 2011 compared with 800 tons for humans. Sweden banned their use in 1986 and Denmark started cutting down drastically in 1994, now using 60% less.[37] In the Netherlands, the use of antibiotics to treat diseases increased after the ban on its use for growth purposes in 2006.[38]
In 2011, the European Parliament voted for a non-binding resolution that called for the end of pre-emptive use of antibiotics in livestock.[39]
A revised regulation on veterinary medicinal products, proposed in procedure 2014/0257/COD, would limit the use of antibiotics in prophylaxis and metaphylaxis. An agreement on the regulation between the Council of the European Union and the European Parliament was confirmed on 13 June 2018.[40][41]
India
In 2011 the Indian government proposed a "National policy for containment of antimicrobial resistance".[42] Other policies set schedules for requiring that food producing animals not be given antibiotics for a certain amount of time before their food goes to market.[43][44] A study released by Centre for Science and Environment (CSE) on 30 July 2014 found antibiotic residues in chicken. This study claims that Indians are developing resistance to antibiotics – and hence falling prey to a host of otherwise curable ailments. Some of this resistance might be due to large-scale unregulated use of antibiotics in the poultry industry. CSE finds that India has not set any limits for antibiotic residues in chicken and says that India will have to implement a comprehensive set of regulations including banning of antibiotic use as growth promoters in the poultry industry. Not doing this will put lives of people at risk.[45]
New Zealand
In 1999 the New Zealand government issued a statement that they would not then ban the use of antibiotics in livestock production.[46] In 2007 ABC Online reported on antibiotic use in chicken production in New Zealand.[47] In 2017, New Zealand published a new action plan to address the ongoing concern of antimicrobial resistance (AMR). The action plan outlined five objectives with each objective looking both at AMR in humans and AMR in agriculture.[48] Compared to other countries, New Zealand has a very low prevalence of AMR in animals and plants. This is due to their low use of antibiotics in animal treatment.[49]
South Korea
In 1998 some researchers reported use in livestock production was a factor in the high prevalence of antibiotic-resistant bacteria in Korea.[50] In 2007 The Korea Times noted that Korea has relatively high usage of antibiotics in livestock production.[51] In 2011, the Korean government banned the use of antibiotics as growth promoters in livestock.[52]
United Kingdom
More than one third of all antibiotics sold in the UK are used on farmed animals.[53]
United States
In 1970 the FDA started recommending that antibiotic use in livestock be limited but set no actual regulations governing this recommendation.[1] By 2001, the Union of Concerned Scientists estimated that greater than 70% of the antibiotics used in the US are given to food animals (for example, chickens, pigs, and cattle), in the absence of disease.[54][55]
In 2004 the Government Accountability Office (GAO) heavily critiqued the FDA for not collecting enough information and data on antibiotic use in factory farms. From this, the GAO concluded that the FDA does not have enough information to create effective policy changes regarding antibiotic use. In response, the FDA insisted that more research was being conducted and voluntary efforts within the industry would solve the problem of antibiotic resistance.[56]
Few policies exist that limit antibiotic use on farms, and some proposed legislation in the US has failed to be adopted.[57] In 2007, two federal bills (S. 549[58] and H.R. 962[59]) aimed to phase out nontherapeutic antibiotics in US food animal production. The Senate bill, introduced by Senator Ted Kennedy, died. The House bill, introduced by Representative Louise Slaughter, died after being referred to Committee. The US Animal Drug User Fee Act was passed by Congress in 2008, requiring that drug manufacturers report all sales of antibiotics into the food animal production industry.[60][61]
By 2011, a total of 13.6 million kg (30 million lb) of antimicrobials were sold for use in food-producing animals in the United States,[62] which represented 80% of all antibiotics sold or distributed in the United States.[63] Of the antibiotics given to animals from 2009 through 2013, just above 60% distributed for food animal use are "medically-important" drugs that are also used in humans.[62] The rest were drug classes like ionophores, which are not used in human medicine.[64]
In March 2012, the United States District Court for the Southern District of New York, ruling in an action brought by the Natural Resources Defense Council and others, ordered the FDA to revoke approvals for the use of antibiotics in livestock that violated FDA regulations.[65] On 11 April 2012 the FDA announced a voluntary program to phase out unsupervised use of drugs as feed additives and convert approved over-the-counter uses for antibiotics to prescription use only, requiring veterinarian supervision of their use and a prescription.[66][67] In December 2013, the FDA announced the commencement of these steps to phase out the use of antibiotics for the purposes of promoting livestock growth.[54][68]
Numerous state senators and members of congress showed support for the Preservation of Antibiotics for Medical Treatment Act of 2013 and Preventing Antibiotic Resistance Act of 2015. These acts proposed amendments be made to the Federal Food, Drug and Cosmetic Act which would limit and preserve the use of antibiotics for medically necessary situations. Both of these bills died in Congress in 2015.[69]
In 2015, the FDA approved a new Veterinary Feed Directive (VFD), which is an updated guideline that give instructions to pharmaceutical companies, veterinarians, and producers about how to administer necessary drugs through the animal's feed and water.[70] The FDA has asked drug companies to voluntarily edit its labels to exclude growth promotion as an indication for antibiotic usage.
The FDA reports that “Under Guidance for Industry (GFI) #213, which went into effect Jan 1, 2017, antibiotics that are important for human medicine can no longer be used for growth promotion or feed efficiency in cows, pigs, chickens, turkeys, and other food animals.”[71]
These new 2017 guidelines would for instance prohibit using a drug off-label for non-therapeutic purposes, which would make using the re-labeled drug for growth enhancement illegal. In addition, some drugs would also be reclassified from OTC to VFD; VFD drugs would require a veterinarian's authorization before it could be delivered in feed.[2][3][72][70] As a result, the FDA report shows a 33% decrease from 2016 to 2017 in domestic sales of medically important antibiotics for use in livestock, but despite this progress the Natural Resources Defense Council (NRDC) remains concerned that beef and pork industries’ sales remain elevated compared to the poultry industries in 2017, and their use is primarily in preventing diseases in healthy animals which further increases the threat on antibiotic resistance.[73]
The key aspect of FDA’s strategy is the request that animal drug sponsors (those who own the right to market the product) voluntarily work with FDA to revise the approved use conditions for their medically important antimicrobial drug products to remove production uses (such as growth enhancement or feed efficiency), and bring the remaining therapeutic uses under veterinary oversight. Once manufacturers voluntarily make these changes, products can no longer be used for production purposes and therapeutic use of these products would require veterinary oversight.[70]
Because of concerns about antibiotics residues getting into the milk or meat of cattle, in the United States, the government requires a withdraw period for any animal treated with antibiotics before it can be slaughtered, to allow residue to exit the animal.[74]
Private entities
Some grocery stores have policies about voluntarily not selling meat produced by using antibiotics to stimulate growth. In response to consumer concerns about the use of antibiotics in poultry, Perdue removed all human antibiotics from its feed in 2007 and launched the Harvestland brand, under which it sold products that met the requirements for an "antibiotic-free" label. By 2014, Perdue had also phased out ionophores from its hatchery and began using the "antibiotic free" labels on its Harvestland, Simply Smart, and Perfect Portions products.[75] By 2015, 52% of the company's chickens were raised without the use of any type of antibiotics.[76]
In 2012 in the United States advocacy organization Consumers Union organized a petition asking the store Trader Joe's to discontinue the sale of meat produced with antibiotics.[77]
Antibiotic resistance
Antimicrobial resistance (AMR) can occur when antibiotics are present in concentrations too low to inhibit bacterial growth, triggering cellular responses in the bacteria that allow them to survive. These bacteria can then reproduce and spread their AMR genes to other generations, increasing their prevalence and leading to infections that cannot be healed by antibiotics.[78]
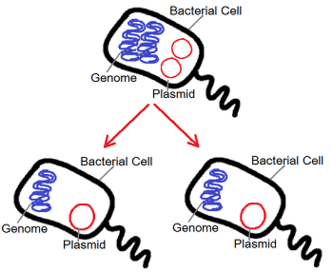
Bacteria can alter their genetic inheritance through two main ways, either by mutating their genetic material or acquiring a new one from other bacteria. The latter being the most important for causing antibiotic-resistant bacteria strains in animals and humans. One of the methods bacteria can obtain new genes is through a process called conjugation which deals with transferring genes using plasmids. These conjugative plasmids carry a number of genes that can be assembled and rearranged, which could then enable bacteria to exchange beneficial genes among themselves ensuring their survival against antibiotics and rendering them ineffective to treat dangerous diseases in humans, resulting into multidrug resistant organisms.[79]
The use of antibiotics in livestock can bring antibiotic-resistant bacteria to humans via consumption of meat and ingestion through airborne bacteria. Manure from food-producing animals can also contain antibiotic-resistant bacteria and is sometimes stored in lagoons. This waste is often sprayed as fertilizer and can thus contaminate crops and water with the bacteria.[80]
Antibiotics are not fully digested and processed in the animal gut; therefore, an estimated 40 to 90% of the antibiotics ingested are excreted in the animal's urine and/or feces.[81] Presence of antibiotics in animal wastes has been widely reported worldwide from multiple farms of different species.[81] Waste from feeding organizations are usually stored on site in large lagoons to be treated before being discharged back into the environment.[81] This composting has been shown to reduce the presence of various antibiotics presence by 20-99%.[81] One study found that chlortetracycline (CTC), an antibiotic used in livestock feed, degraded at different rates dependent on the animal it was fed to.[82] Half-lives of CTC were as high as 86.6 days in hog manure, while only 90% of CTC was depleted after 42 of composting for other animals.[82] The researchers concluded that manure composting was not sufficient to ensure the microbial degradation of CTC.[82] The high amounts of antibiotics present in animal waste, even after long periods of composting, are a potential pollution source of environmental antibiotics.[81] Animal waste is often recycled and used as fertilizer for crops.[83] Consequently, antibiotics, while small amounts, have been found in crops grown in these fertilized fields.[84] In another study, antibiotics were detected in runoff from animal waste fertilized lands at concentrations up to 9 nanograms per liter.[85] In some cases, animal wastes are directly released to receiving watersheds through intentional discharge or leaching from farms.[81]
.jpg)
Waste can also be released into the environment in cases of flooding. North Carolina's hog farms produces 10 billion gallons of manure annually.[14] In 1999, Hurricane Floyd caused lagoon spillovers at 46 farms in North Carolina.[86] Fifteen years later, a study done by the NIH showed that fecal bacteria concentrations were still high in surface waters both up and downstream from the farms.[86] Forty percent of the samples collected exceeded state and federal guidelines for concentrations of E. coli.[86] In preparation for the 2018 Hurricane Florence, hog farmers attempted to reduce the volume of their manure lagoons by spraying it onto surrounding fields, which, according to environmental experts, could runoff into water sources after the flooding caused by Florence.[14] By the end of Hurricane Florence, 26 hog manure lagoons had been damaged and 13 overflowed - meaning their contents spilled over into the floodwater.[87]
The World Health Organization (WHO) has published a revised list in 2017, "Critically Important Antimicrobials for Human Medicine, 5th revision", with the intent that it be used "as a reference to help formulate and prioritize risk assessment and risk management strategies for containing antimicrobial resistance due to human and non-human antimicrobial use to help preserve the effectiveness of currently available antimicrobials.[88]
Effects in humans
The effects of antibiotic usage in livestock transferring to humans has been well documented for over 40 years.[18] It was first documented in 1976, where a study followed a novel antibiotic being used in livestock. The bacteria in animals and workers were regularly followed to record translational effects. The findings revealed that within 2 weeks, the bacteria found in the guts of animals fed antibiotics were resistant to the new antibiotic.[89] Additionally, the resistant bacteria had spread to farm laborers within six months. The bacteria in the stool of the laborers were tested and contained more than 80% resistance to the initial antibiotic given to the livestock.[19] Since the primary study, there have been many well-documented events showing that antibiotic usage in livestock results in direct influence of antibiotic resistance in humans.
In 2017, the WHO included methicillin-resistant S. aureus (MRSA) in its priority list of 12 antibiotic-resistant bacteria, urging the need to search for new and more effective antibiotics against it. There also has been an increase in the number of bacterial pathogens resistant to multiple antimicrobial agents, including MRSA, which have recently emerged into different lineages. Some of them are associated with livestock and companion animals that are then able to be transmitted to humans, also called livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA). These new lineages have caused the infections in exposed humans to be difficult to treat and have become a public health concern.[90]
A study looked at the association between exposure to livestock and the occurrence of livestock-associated MRSA (LA-MRSA) infection and observed that LA-MRSA infection was 9.64 times as likely to be found among livestock workers and veterinarians compared to their unexposed families and community members, showing that exposure to livestock significantly increases the risk of developing methicillin-resistant Staphylococcus aureus (MRSA) infection which has now become an increasing public health concern worldwide.[91]
Another study found that 45.5% of industrial hog operation workers still carried in their nostrils livestock-associated Staphylococcus aureus (LA-SA) over a 14-day period up to 96h away from work, which included workers who were persistent carriers of livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) and livestock-associated multidrug-resistant Staphylococcus aureus (LA-MDRSA), showing that industrial hog operation workers are therefore at an increased risk in developing infections in clinical settings.[92]
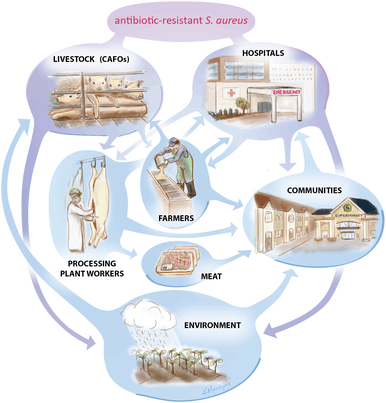
The Center for Disease Control identifies Salmonella and Campylobacter as two bacteria commonly spread to humans through food.[93] According to the 2013 AR Threats Report, these two bacteria alone account for over 400,000 Americans becoming sick from antibiotic-resistant infections every year.[93] When antibiotics are used for animal health, resistant bacteria can still survive and contaminate animal products during processing and slaughtering. The resistant bacteria can also be transferred to the environment through soils and water via animal manure.[93] When the contaminated soil and water is used to fertilize crops like fruits and vegetables, then resistant bacteria is spread further to the human population.[93]
Major bacterial infections in humans can be traced back to livestock. The family Enterobacteriaceae includes many opportunistic bacteria, including E. coli,[89] which are commonly found in livestock. Other bacteria include Klebsiella and Staphylococcus aureus. They cause infections in the urinary tract, digestive system, skin, and bloodstream, and account for a significant portion of antibiotic-resistant bacterial infections.[89] Some ways to prevent the spread of antibiotic resistant bacteria to humans is ensuring hands are clean, hands are always properly washed with soap after touching any raw foods/livestock, and are scrubbed for at least 15 seconds.[94]
Advocates for restricting antibiotic use
Antibiotic use in animals is approved for treatment of sick animals. The FDA has approved responsible use of antibiotics to control disease spread in a population of animals when members of the population are sick, prevent spread of disease for at-risk animals, and treat infected animals.[93] However, the CDC and FDA do not support the use of antibiotics for growth promotion because of evidence that suggests antibiotics for growth promotion lead to the development of resistant bacteria.[93]
The practice of using antibiotics for growth stimulation is problematic for these reasons:[95]
- It is the largest use of antimicrobials worldwide
- Subtherapeutic use of antibiotics results in bacterial resistance
- Every important class of antibiotics are being used in this way, making every class less effective
- The bacteria being changed harm humans
Donald Kennedy, former director of the FDA, has said "There's no question that routinely administering non-therapeutic doses of antibiotics to food animals contributes to antibiotic resistance."[96] David Aaron Kessler, another former director, stated, "We have more than enough scientific evidence to justify curbing the rampant use of antibiotics for livestock, yet the food and drug industries are not only fighting proposed legislation to reduce these practices, they also oppose collecting the data."[97]
In 2013, the US Centers for Disease Control and Prevention (CDC) published a white paper discussing antibiotic resistance threats in the US and calling for "improved use of antibiotics" among other measures to contain the threat to human health. The CDC asked leaders in agriculture, healthcare, and other disciplines to work together to combat the issue of increasing antibiotic resistance.[98]
Some scientists have said that "all therapeutic antimicrobial agents should be available only by prescription for human and veterinary use."[99]
The Pew Charitable Trusts have stated that "hundreds of scientific studies conducted over four decades demonstrate that feeding low doses of antibiotics to livestock breeds antibiotic-resistant superbugs that can infect people. The FDA, the U.S. Department of Agriculture and the Centers for Disease Control and Prevention all testified before Congress that there is a definitive link between the routine, non-therapeutic use of antibiotics in food animal production and the challenge of antibiotic resistance in humans."[100]
In 2017, the World Health Organization (WHO) recommended reducing antibiotic use in animals used in the food industry. Due to the increasing risk of antibiotic resistant bacteria, the WHO strongly suggested restrictions on antibiotics being used for growth promotion and antibiotics used on healthy animals. Animals that require antibiotics should be treated with antibiotics that pose the smallest risk to human health.[101]
HSBC produced a report in October 2018 warning that the use of antibiotics in meat production could have “devastating” consequences for humans. It noted that many dairy and meat producers in Asia and the Americas had an economic incentive to continue high usage of antibiotics, particularly in crowded or unsanitary living conditions.[102]
Moderate positions
The World Organisation for Animal Health has acknowledged the need to protect antibiotics but argued against a total ban on antibiotic use in animal production.[103]
A total ban on antibiotics might drastically reduce protein supply in some parts of the world.[101]
Advocates for status quo
In 2011 the National Pork Producers Council, an American trade association, has said, "Not only is there no scientific study linking antibiotic use in food animals to antibiotic resistance in humans, as the U.S. pork industry has continually pointed out, but there isn't even adequate data to conduct a study."[104] The statement contradicts scientific consensus,[105] and was issued in response to a United States Government Accountability Office report that asserts, "Antibiotic use in food animals contributes to the emergence of resistant bacteria that may affect humans".[106]
The National Pork Board, a government-owned corporation of the United States, has said, "The vast majority of producers use (antibiotics) appropriately."[107]
Effects of restricting antibiotic use
When government regulation restricts use of antibiotics, the negative economic impact is not often considered.[108]
When antibiotics are used subtheraputically (for animal performance, increased growth, and improved feed efficiency), then the costs of meat, eggs, and other animal products are lowered.[109] One big argument against the restriction of antibiotic use is the potential economic hardship that would result for producers of livestock and poultry that could also result in higher cost for consumers. In a study analysing the economic cost of the FDA restricting all antibiotic use in animal livestock, it was estimated that the restriction would cost consumers approximately $1.2 billion to $2.5 billion per year.[109] In order to determine the overall economic impact of restricting antibiotic use, the financial cost must be weighed against the health benefits to the population. Since it is difficult to estimate quantifications of potential health benefits, the study concluded that the complete economic impact of restricting antibiotic use has not yet been determined.[109]
Although quantifying health benefits may be difficult, the economic impact of antibiotic restriction in animals can also be evaluated through the economic impact of antibiotic resistance in humans, which is a significant outcome of antibiotic use in animals. The World Health Organization identifies antibiotic resistance as a contributor to longer hospital stays and higher medical costs.[110] When infections can no longer be treated by typical first-line antibiotics, more expensive medications are required for treatment. When illness duration is extended by antibiotics resistance, the increased health care costs create a larger economic burden for families and societies.[110] The Center for Infectious Disease Research and Policy estimates approximately $2.2 billion in antibiotic resistance- related healthcare costs each year.[111]
While restricting antibiotics in animals causes a significant economic burden, the outcome of antibiotic resistance in humans that is perpetuated by antibiotic use in animals carries a comparable economic burden.
Regulation of antibiotics in livestock production would affect the business models of corporations such as Tyson Foods, Cargill, and Hormel.[112]
Difficulties with determining relevant facts
It is difficult to set up a comprehensive surveillance system for measuring rates of change in antibiotic resistance.[113] The US Government Accountability Office published a report in 2011 stating that government and commercial agencies had not been collecting sufficient data to make a decision about best practices.[114]
Currently, there is no regulatory agency in the United States that systematically collects detailed data on antibiotic use in humans and animals. It is not clear which antibiotics are prescribed for which purpose and at what time. Furthermore, the world has no surveillance infrastructure to monitor emerging antibiotic resistance threats. Because of these issues, it is difficult to quantify antibiotic resistance, to regulate antibiotic prescribing practices, and to detect and respond to rising threats.[98]
Specific resistance that has been identified
At this time, the most well-documented impact on humans is foodborne gastrointestinal illness. In most cases, these illnesses are mild and do not require antibiotics; though if the infectious bacteria is drug-resistant, they have increased virulence (ability to cause disease) and lead to prolonged illness. Furthermore, in approximately 10% of cases, the disease becomes severe, requiring more advanced treatments. These treatments can take the form of intravenous antibiotics, supportive care for blood infections, and hospital stays, leading to higher costs and greater morbidity, with a trend toward higher mortality.[98][115] Though all people are susceptible, populations at higher risk for severe disease include children, the elderly, and those with chronic disease.[98][116][117]
Over the past 20 years, the most common drug-resistant foodborne bacteria in industrialized countries have been non-typhoidal Salmonella and Campylobacter. Research has consistently shown the main contributing factors are bacteria sourced in livestock.[115] A 1998 outbreak of multidrug-resistant Salmonella in Denmark linked back to two Danish swine herds.[116] Coupled with the discovery of this link, there have been improved monitoring systems that have helped to quantify the impact. In the United States, it is estimated that there are approximately 400,000 cases and over 35,000 hospitalizations per year attributable to increasing resistant strains of Salmonella and Campylobacter. In terms of financial impact in the US, the treatment of non-typhoidal Salmonella infections alone is now estimated to cost $365 million per year.[98][118] In light of this, in its inaugural 2013 report on antibiotic resistance threats in the United States, the CDC identified resistant non-typhoidal Salmonella and Campylobacter as "serious threats" and called for improved surveillance and intervention in food production moving forward.[98]
There are other bacteria as well, where research is evolving and revealing that bacterial resistance acquired through use in livestock may be contributing to disease in humans. Examples of these include Enterococcus (including E. coli 0157) and Staphylococcus aureus. For foodborne illness from E. coli, which is still not typically treated with antibiotics because of associated risk of renal failure, increasing rates of antibiotic-resistant infections have been correlated with increasing virulence of the bacteria.[118][119] In the case of Enterococcus and S. aureus, resistant forms of both of these bacteria have resulted in greatly increasing morbidity and mortality in the US.[98] At this point, there have been studies, though a limited number, that definitively link antibiotic use in food production to these resistance patterns in humans and further research will help to further characterize this relationship.[117][120][121]
Mechanisms for transfer to humans
Antibiotics given in concentrations too low to combat disease are called “subtherapeutic.” The administration of these drugs when there is no diagnosis of disease result in decreased mortality and morbidity and increased growth in the animals treated. It is theorized that subtherapeutic doses kill some, but not all, of the bacterial organisms in the animal – likely leaving those that are naturally antibiotic-resistant.[122] The actual mechanism by which subtherapeutic antibiotic feed additives serve as growth promoters is still unclear. Some people have speculated that animals and fowl may have subclinical infections, which would be cured by low levels of antibiotics in feed, thereby allowing the animals to thrive.
Humans can be exposed to antibiotic-resistant bacteria by ingesting them through the food supply. Dairy products, ground beef, and poultry are the most common foods harboring these pathogens.[123] There is evidence that a large proportion of resistant E. coli isolates causing bloodstream infections in people are from livestock produced as food.[124]
When manure from antibiotic-fed swine is used as fertilizer elsewhere, the manure may be contaminated with bacteria which can infect humans.[125]
Studies have also shown that direct contact with livestock can lead to the spread of antibiotic-resistant bacteria from animals to humans.[126][127]
Sampling of retail meats such as turkey, chicken, pork and beef consistently show high levels of Enterobacteriaceae. Rates of resistant bacteria in these meats are high as well. Sources on contaminated meat put humans at direct risk by handling the meat or ingesting it before it is completely cooked.[89] Food preservation methods can help eliminate, decrease, or prevent the growth of bacteria. Evidence for the transfer of macrolide-resistant microorganisms from animals to humans has been scant, and most evidence shows that pathogens of concern in human populations originated in humans and are maintained there, with rare cases of transference to humans.[128][129]
Research into alternatives
Increasing concern due to the emergence of antibiotic-resistant bacteria has led researchers to look for alternatives to using antibiotics in livestock.[130]
Probiotics, cultures of a single bacteria strain or mixture of different strains, are being studied in livestock as a production enhancer.[131]
Prebiotics are non-digestible carbohydrates. The carbohydrates are mainly made up of oligosaccharides which are short chains of monosaccharides. The two most commonly studied prebiotics are fructooligosaccharides (FOS) and mannanoligosaccharides (MOS). FOS has been studied for use in chicken feed. MOS works as a competitive binding site, as bacteria bind to it rather than the intestine and are carried out.[132]
Bacteriophages are able to infect most bacteria and are easily found in most environments colonized by bacteria, and have been studied as well.[130]
In another study it was found that using probiotics, competitive exclusion, enzymes, immunomodulators and organic acids prevents the spread of bacteria and can all be used in place of antibiotics.[133] Another research team was able to use bacteriocins, antimicrobial peptides and bacteriophages in the control of bacterial infections.[134] While further research is needed in this field, alternative methods have been identified in effectively controlling bacterial infections in animals. All of the alternative methods listed pose no known threat to human health and all can lead the elimination of antibiotics in factory farms. With further research it is highly likely that a cost effective and health effective alternative could and will be found.
Other alternatives include preventative approaches to keep the animals healthy to eliminate the need for antibiotics. These alternatives include improving the living conditions for animals, better hygiene practices and more efficient vaccinations.[101]
See also
- Antibiotic misuse
- Veganism
- Environmental impact of meat production
- List of antibiotic-resistant bacteria
References
- "The Judicious Use of Medically Important Antimicrobial Drugs in Food-Producing Animals" (PDF). Guidance for Industry (#209). 2012.
- "Veterinary Feed Directive (VFD) Basics". AVMA. Retrieved 14 March 2017.
- University of Nebraska, Lincoln (October 2015). "Veterinary Feed Directive Questions and Answers". UNL Beef. Retrieved 14 March 2017.
- Ogle, Maureen (3 September 2013). "Riots, Rage and Resistance: A Brief History of How Antibiotics Arrived on the Farm". Scientific American. Retrieved 5 November 2013.
- Reported locally in these:
- "To Become Vegetarians", Mansfield (O.) News, 17 January 1910, p2
- "150,000 at Cleveland Stop the Use of Meat" Syracuse Herald-Journal, 25 January 1910, p1
- "Boycott on Meat is Rapidly Spreading; Men Who Are Blamed For High Price", Atlanta Constitution, 25 January 1910, p1
- "A review of hot and sweet pepper added in animal nutrition: alternative against the use of antibiotics | Request PDF". ResearchGate. Retrieved 26 March 2019.
- "Hogging It!: Estimates of Antimicrobial Abuse in Livestock". Union of Concerned Scientists. 2001.
- Reinhardt, Christopher. "Antimicrobial Feed Additives". Merck Veterinary Manual.
- Allen, Heather K.; Stanton, Thad B. (1 January 2014). "Altered egos: antibiotic effects on food animal microbiomes". Annual Review of Microbiology. 68: 297–315. doi:10.1146/annurev-micro-091213-113052. ISSN 1545-3251. PMID 25002091.
- Reinhardt, Christopher D. (2012), "Antimicrobial Feed Additives", in Aiello, Susan E.; Moses, Michael A. (eds.), Merck Veterinary Manual, Merck & Co. and Merial
- "Growth Promotion — Antimicrobial Resistance Learning Site For Veterinary Students". amrls.cvm.msu.edu. Retrieved 22 June 2018.
- CAFOS- Victims without a voice. (2011). SearcyLaw. Retrieved from https://www.searcylaw.com/cafos-victims-without-a-voice/
- Nicole, Wendee (1 June 2013). "CAFOs and Environmental Justice: The Case of North Carolina". Environmental Health Perspectives. 121 (6): a182–a189. doi:10.1289/ehp.121-a182. ISSN 0091-6765. PMC 3672924. PMID 23732659.
- Pierre Louis, K (19 September 2018). "Lagoons of Pig Waste Are Overflowing After Florence. Yes, That's as Nasty as It Sounds". The New York Times.
- Hribar, Carrie (2010). "Understanding Concentrated Animal Feeding Operations and Their Impact on Communities" (PDF). National Association of Local Boards of Health.
- Lashment, Tiffany (15 November 2017). "Watch for "Absolute Pollution" Clause". Dairyherd. Retrieved 23 June 2018.
- "The Quality of the Nation's Groundwater". www.usgs.gov. Retrieved 26 March 2019.
- Ventola, C. Lee (April 2015). "The Antibiotic Resistance Crisis". Pharmacy and Therapeutics. 40 (4): 277–283. ISSN 1052-1372. PMC 4378521. PMID 25859123.
- Marshall, Bonnie M.; Levy, Stuart B. (October 2011). "Food Animals and Antimicrobials: Impacts on Human Health". Clinical Microbiology Reviews. 24 (4): 718–733. doi:10.1128/CMR.00002-11. ISSN 0893-8512. PMC 3194830. PMID 21976606.
- CDC (10 September 2018). "Antibiotic Resistance and Food are Connected". Centers for Disease Control and Prevention. Retrieved 26 March 2019.
- Zurek, Ludek; Ghosh, Anuradha (June 2014). "Insects Represent a Link between Food Animal Farms and the Urban Environment for Antibiotic Resistance Traits". Applied and Environmental Microbiology. 80 (12): 3562–3567. doi:10.1128/AEM.00600-14. ISSN 0099-2240. PMC 4054130. PMID 24705326.
- Millen, D. D.; Pacheco, R. D. L.; Meyer, P. M.; Rodrigues, P. H. M.; De Beni Arrigoni, M. (2011). "Current outlook and future perspectives of beef production in Brazil". Animal Frontiers. 1 (2): 46–52. doi:10.2527/af.2011-0017.
- Canadian Cattlemen's Association and Beef Information Centre (2003). "Understanding Use of Antibiotic and Hormonal Substances in Beef Cattle". Nutrition Perspective. Archived from the original on May 17, 2016. Retrieved October 29, 2009.
- Tatlow, Didi Kirsten (18 February 2013). "Global Health Threat Seen in Overuse of Antibiotics on Chinese Pig Farms". IHT Rendezvous. Retrieved 28 August 2013.
- Wei, R.; Ge, F.; Huang, S.; Chen, M.; Wang, R. (2011). "Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China" (PDF). Chemosphere. 82 (10): 1408–1414. Bibcode:2011Chmsp..82.1408W. doi:10.1016/j.chemosphere.2010.11.067. PMID 21159362. Archived from the original (PDF) on 17 August 2018. Retrieved 17 August 2018.
- Hu, X.; Zhou, Q.; Luo, Y. (2010). "Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China". Environmental Pollution. 158 (9): 2992–2998. doi:10.1016/j.envpol.2010.05.023. PMID 20580472.
- Zhao, L.; Dong, Y. H.; Wang, H. (2010). "Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China". Science of the Total Environment. 408 (5): 1069–1075. Bibcode:2010ScTEn.408.1069Z. doi:10.1016/j.scitotenv.2009.11.014. PMID 19954821.
- Krishnasamy, Vikram; Otte, Joachim; Silbergeld, Ellen (28 April 2015). "Antimicrobial use in Chinese swine and broiler poultry production". Antimicrobial Resistance and Infection Control. 4 (1): 17. doi:10.1186/s13756-015-0050-y. ISSN 2047-2994. PMC 4412119. PMID 25922664.
- Mu, Quanhua; Li, Jin; Sun, Yingxue; Mao, Daqing; Wang, Qing; Yi, Luo (5 December 2014). "Occurrence of sulfonamide-, tetracycline-, plasmid-mediated quinolone- and macrolide-resistance genes in livestock feedlots in Northern China". Springer. 22 (6932–6940): 6932–6940. doi:10.1007/s11356-014-3905-5. PMID 25475616.
- Salamon, Maureen (11 February 2013). "China's Overuse of Antibiotics in Livestock May Threaten Human Health". health.usnews.com. Retrieved 28 August 2013.
- https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/662189/UK_AMR_3rd_annual_report.pdf
- Medical Research Council, M. R. C. (29 November 2018). "£4.5m from Newton Fund for collaborations that will tackle antimicrobial resistance". mrc.ukri.org. Retrieved 6 December 2018.
- "农业部关于印发《全国遏制动物源细菌耐药行动计划(2017—2020年)》的通知". jiuban.moa.gov.cn. Retrieved 6 December 2018.
- "A National Action Plan to Contain Antimicrobial Resistance in China: Contents, Actions and Expectations | AMR Control". resistancecontrol.info. Retrieved 6 December 2018.
- European Union (2005). "European Commission - PRESS RELEASES - Press release - Ban on antibiotics as growth promoters in animal feed enters into effect". Retrieved 22 December 2005.
- "EUR-Lex - 32003R1831 - EN - EUR-Lex". data.europa.eu.
- Koch, Julia (13 November 2013). "Cutting Antibiotics : Denmark Leads Way in Healthier Pig Farming". Spiegel Online. Retrieved 22 May 2014.
- Cogliani, Carol; Goossens, Herman; Greko, Christina (1 January 2011). "Restricting Antimicrobial Use in Food Animals: Lessons from Europe". Microbe Magazine. 6 (6): 274–279. doi:10.1128/microbe.6.274.1. ISSN 1558-7452.
- "Parliament demands smarter use of antibiotics". www.europarl.europa.eu.
- "EUR-Lex - 2014_257 - EN - EUR-Lex". eur-lex.europa.eu.
- "Veterinary medicines: new EU rules to enhance availability and fight against antimicrobial resistance - Consilium". www.consilium.europa.eu.
- Thacker, Teena (13 April 2011). "Govt wants to limit use of antibiotics in animals – Indian Express". indianexpress.com. Retrieved 28 August 2013.
- Sinha, Kounteya (25 November 2011). "New norm to curb antibiotic resistance – Times of India". indiatimes.com. Retrieved 28 August 2013.
- Sinha, Kounteya (6 April 2012). "In a first, antibiotics bar on food-producing animals – Times of India". indiatimes.com. Retrieved 28 August 2013.
- "Centre For Science and Environment (CSE)". 30 July 2014. Retrieved 30 July 2014.
- staff (7 January 1999). "NZ holds off ban on animal antibiotics – National – NZ Herald News". nzherald.co.nz. Retrieved 29 August 2013.
- Williams, Robyn; Cook, Greg (11 August 2007). "Antibiotics and intensive chicken farming in New Zealand – The Science Show". abc.net.au. Retrieved 29 August 2013.
- Ministry of Health and Ministry for Primary Industries. 2017. Antimicrobial Resistance: New Zealand’s current situation and identified areas for action. Wellington: Ministry of Health and Ministry for Primary Industries. Retrieved from https://www.health.govt.nz/publication/antimicrobial-resistance-new-zealands-current-situation-and-identified-areas-action
- Hillerton J., Irvine C., Bryan M., Scott D., Merchant S. (2016). "Use of antimicrobials for animals in New Zealand, and in comparison with other countries". New Zealand Veterinary Journal. 65 (2): 71–77. doi:10.1080/00480169.2016.1171736. PMID 27030313.CS1 maint: multiple names: authors list (link)
- Kim, Woo Joo; Park, Seung Chull (1998). "bacterial Resistance to Antimicrobial Agents: An Overview from Korea" (PDF). Yonsei Medical Journal. 39 (6): 488–494. doi:10.3349/ymj.1998.39.6.488. PMID 10097674. Retrieved 29 August 2013.
- Won-sup, Yoon (25 June 2007). "Antibiotics in Livestock Harm Human Beings". The Korea Times. Retrieved 29 August 2013.
- Flynn, Dan (7 June 2011). "South Korea Bans Antibiotics in Animal Feed". foodsafetynews.com. Retrieved 29 August 2013.
- "UK One Health Report: Joint report on antibiotic use and antibiotic resistance" (PDF). 31 January 2019.
- Martin Khor (18 May 2014). "Why Are Antibiotics Becoming Useless All Over the World?". The Real News. Retrieved 18 May 2014.
- "Executive summary from the UCS report "Hogging It: Estimates of Antimicrobial Abuse in Livestock"". Union of Concerned Scientists. January 2001.
- GAO. 2011: Antibiotic Resistance: Agencies Have Made Limited Progress Addressing Antibiotic Use in Animals. GAO-11-801.
- Philpott, Tom (17 September 2010). "UPDATED: With the food safety bill dead, time for the FDA/USDA to grow some backbone". Grist. Retrieved 27 August 2013.
- "US Senate Bill S. 549: Preservation of Antibiotics for Medical Treatment Act of 2007".
- "Preservation of Antibiotics for Medical Treatment Act of 2007".
- Rogers, Laura (28 December 2012). "Laura Rogers: A New Year's Resolution: Put Animals on an Antibiotics Diet". huffingtonpost.com. Retrieved 26 August 2013.
- Wallinga, David (12 February 2013). "David Wallinga, M.D.: Animal Antibiotic Use Continues Upwards, FDA Keeps Blinders on". huffingtonpost.com. Retrieved 27 August 2013.
- Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals (PDF) (Report). FDA. 2013.
- Drug Use Review (PDF) (Report). FDA. 2012.
- "Evaluating the Safety of Antimicrobial New Animal Drugs with Regard to Their Microbiological Effects on Bacteria of Human Health Concern" (PDF). Guidance for Industry (#152). 2003.
- John Gever (23 March 2012). "FDA Told to Move on Antibiotic Use in Livestock". MedPage Today. Retrieved 24 March 2012.
- Gardiner Harris (11 April 2012). "U.S. Tightens Rules on Antibiotics Use for Livestock". The New York Times. Retrieved 12 April 2012.
- "FDA's Strategy on Antimicrobial Resistance — Questions and Answers". US Food and Drug Administration. 11 April 2012. Retrieved 12 April 2012.
"Judicious use" is using an antimicrobial drug appropriately and only when necessary; Based on a thorough review of the available scientific information, FDA recommends that use of medically important antimicrobial drugs in food-producing animals be limited to situations where the use of these drugs is necessary for ensuring animal health, and their use includes veterinary oversight or consultation. FDA believes that using medically important antimicrobial drugs to increase production in food-producing animals is not a judicious use.
- Tavernise, Sabrina (11 December 2013). "F.D.A. to Phase Out Use of Some Antibiotics in Animals Raised for Meat". The New York Times. Retrieved 11 December 2013.
- Govtrack.us https://www.govtrack.us/congress/bills/113/hr1150
- Center for Veterinary Medicine. "FDA's Strategy on Antimicrobial Resistance - Questions and Answers". Guidance for Industry. Retrieved 14 March 2017.
- Summary Report On Antimicrobials Sold or Distributed for Use in Food-Producing Animals. (2018). US Food and Drug Administration. Retrieved from https://www.fda.gov/downloads/ForIndustry/UserFees/AnimalDrugUserFeeActADUFA/UCM628538.pdf
- Cornell University College of Veterinary Medicine. "VFD – Veterinary Feed Directive". Retrieved 14 March 2017.
- Dall C. (2018). FDA reports major drop in antibiotics for food animals. CIDRAP News. Retrieved from http://www.cidrap.umn.edu/news-perspective/2018/12/fda-reports-major-drop-antibiotics-food-animals
- USDA. "Beef...from Farm to Table". Fact Sheets. Archived from the original on 24 February 2013.
- Stephanie Strom (31 July 2015). "Perdue Sharply Cuts Antibiotic Use in Chickens and Jabs at Its Rivals". The New York Times. Retrieved 12 August 2015.
- "Antibiotics Position Statement". Archived from the original on 28 July 2015. Retrieved 12 August 2015.
- "Meat Without Drugs". Consumers Union. Retrieved 27 August 2013., which is described in the following works
- Gore, Al (2013). "The Reinvention of Life and Death: Antibiotics before Swine". The Future : Six Drivers of Global Change (First ed.). New York: Random House. pp. 227 and citation on 475. ISBN 9780812992946.
- Hurd, Scott (26 June 2012). "Commentary: 'Meat without Drugs' could be inhumane". Bovine Veterinarian. Retrieved 27 August 2013.
All peer-reviewed scientific risk assessments have demonstrated a negligible risk of human health harm due to livestock antibiotic use.
- Greenaway, Twilight (20 June 2012). "Your meat on drugs: Will grocery stores cut out antibiotics?". Grist. Retrieved 27 August 2013.
- Ben, Yujie; Fu, Caixia; Hu, Min; Liu, Lei; Wong, Ming Hung; Zheng, Chunmiao (February 2019). "Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review". Environmental Research. 169: 483–493. Bibcode:2019ER....169..483B. doi:10.1016/j.envres.2018.11.040. ISSN 1096-0953. PMID 30530088.
- Bennett P. M. (2008). Plasmid encoded antibiotic resistance: acquisition and transfer of antibiotic resistance genes in bacteria. British journal of pharmacology, 153 Suppl 1(Suppl 1), S347-57.
- Food & Water Watch (2015). Antibiotic Resistance 101: How How Antibiotic Misuse on Factory Farms Can Make You Sick (PDF) (Report). pp. 1–17.
- Zhang, X (2014). "Prevalence of veterinary antibiotics and antibiotic-resistant Escherichia coli in the surface water of a livestock production region in northern China". PLOS ONE. 9 (11): e111026. Bibcode:2014PLoSO...9k1026Z. doi:10.1371/journal.pone.0111026. PMC 4220964. PMID 25372873.
- Bao, Yanyu; Zhou, Qixing; Guan, Lianzhu; Wang, Yingying (April 2009). "Depletion of chlortetracycline during composting of aged and spiked manures". Waste Management. 29 (4): 1416–1423. doi:10.1016/j.wasman.2008.08.022. PMID 18954968.
- Larsson, D.G. (2014). "Antibiotics in the environment". Upsala Journal of Medical Sciences. 119 (2): 108–112. doi:10.3109/03009734.2014.896438. PMC 4034546. PMID 24646081.
- Cimitile, Matthew. "Worried about Antibiotics in Your Beef? Vegetables May Be No Better".
- Sun, P (2013). "Detection and quantification of ionophore antibiotics in runoff, soil and poultry litter". Journal of Chromatography A. 1312: 10–7. doi:10.1016/j.chroma.2013.08.044. PMID 24028934.
- Helmer, Jodi. "Hurricane-Flooded Hog Farms Could Bring Superbugs to North Carolina Communities".
- Brown, C (18 September 2018). "In North Carolina, losses of nearly 2 million birds and 26 flooded lagoons reported".
- WHO list of critically important antimicrobials (WHO CIA list). Food Safety. World Health Organization. Retrieved from https://www.who.int/foodsafety/areas_work/antimicrobial-resistance/cia/en/
- Economou, Vangelis; Gousia, Panagiota (1 April 2015). "Agriculture and food animals as a source of antimicrobial-resistant bacteria". Infection and Drug Resistance. 8: 49–61. doi:10.2147/IDR.S55778. ISSN 1178-6973. PMC 4388096. PMID 25878509.
- Livestock-associated Staphylococcus Aureus (LA-MRSA), Research topic. Retrieved from: https://www.frontiersin.org/research-topics/6689/livestock-associated-staphylococcus-aureus-la-mrsa
- Chen, C., & Wu, F. (2018). Livestock-Associated Methicillin-Resistant Staphylococcus Aureus (LA-MRSA) Colonization and Infection Among Livestock Workers and Veterinarians: A Systematic Review and Meta-Analysis. Available at SSRN 3208968.
- Nadimpalli M., Rinsky J. L., Wing S., Hall D., Stewart J., Larsen J., Strelitz J. (2015). "Persistence of livestock-associated antibiotic-resistant Staphylococcus aureus among industrial hog operation workers in North Carolina over 14 days". Occup Environ Med. 72 (2): 90–99. doi:10.1136/oemed-2014-102095. PMC 4316926. PMID 25200855.CS1 maint: multiple names: authors list (link)
- CDC (10 September 2018). "Antibiotic Resistance and Food are Connected". Centers for Disease Control and Prevention. Retrieved 29 March 2019.
- Rajendran, Reshma (2018). "Superbug infection". World Journal of Pharmaceutical Research. 7: 275–287. doi:10.20959/wjpr2018-11480 (inactive 11 November 2019).
- Silbergeld, E. K.; Graham, J.; Price, L. B. (2008). "Industrial Food Animal Production, Antimicrobial Resistance, and Human Health". Annual Review of Public Health. 29: 151–169. doi:10.1146/annurev.publhealth.29.020907.090904. PMID 18348709.
- McVeigh, Karen (19 September 2012). "Scientists: overuse of antibiotics in animal agriculture endangers humans". The Guardian. ISSN 0261-3077. Retrieved 23 August 2013.
- Kessler, David Aaron (27 March 2013). "Antibiotics and the Meat We Eat". The New York Times. New York City: NYTC. ISSN 0362-4331. Retrieved 26 August 2013.
- "Antibiotic Resistance Threats in the United States" (PDF). Centers for Disease Control and Prevention. Retrieved 30 December 2016.
- Gilchrist, M. J.; Greko, C.; Wallinga, D. B.; Beran, G. W.; Riley, D. G.; Thorne, P. S. (2006). "The Potential Role of Concentrated Animal Feeding Operations in Infectious Disease Epidemics and Antibiotic Resistance". Environmental Health Perspectives. 115 (2): 313–316. doi:10.1289/ehp.8837. PMC 1817683. PMID 17384785.
- The Pew Charitable Trusts (15 October 2012). "Pew Comments on Proposed Antibiotics Legislation". Retrieved 26 August 2013.
- Stop using antibiotics in healthy animals to preserve their effectiveness. (2017). Retrieved from https://www.who.int/news-room/detail/07-11-2017-stop-using-antibiotics-in-healthy-animals-to-prevent-the-spread-of-antibiotic-resistance
- "One of the world's largest banks has issued an alarming warning about antibiotic resistance — with big consequences for humanity". Business Insider UK. 10 October 2018. Retrieved 13 November 2018.
- de La Hamaide, Sybille (11 January 2012). "Antibiotics for livestock vital to feed world: OIE | Reuters". reuters.com. Retrieved 28 August 2013.
-
- National Pork Producers Council (15 September 2011). "Nat'l Pork Producers Council Issues Statement About GAO's Report on Antibiotic Resistance". growinggeorgia.com. Retrieved 29 August 2013.
- Philpott, Tom (21 September 2011). "Meat Industry Still Denying Antibiotic Resistance". motherjones.com. Retrieved 29 August 2013.
- "Antibiotic Resistance and Food Animal Production: a Bibliography of Scientific Studies (1969-2012)" (PDF). The Pew Charitable Trusts. Archived from the original (PDF) on 11 August 2012.
- Office, U. S. Government Accountability (7 September 2011). "Antibiotic Resistance: Agencies Have Made Limited Progress Addressing Antibiotic Use in Animals" (GAO-11–801). U.S. Government Accountability Office.
Antibiotics have saved millions of lives, but antibiotic use in food animals contributes to the emergence of resistant bacteria that may affect humans.
Cite journal requires|journal=(help) - Couric, Katie (10 February 2010). "Animal Antibiotic Overuse Hurting Humans?". CBS News. New York City: CBS. Retrieved 29 August 2013.
- Phillips, I.; Casewell, M.; Cox, T.; De Groot, B.; Friis, C.; Jones, R.; Nightingale, C.; Preston, R.; Waddell, J. (2003). "Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data". Journal of Antimicrobial Chemotherapy. 53 (1): 28–52. doi:10.1093/jac/dkg483. PMID 14657094.
- National Research Council (1999). "The Use of Drugs in Food Animals: Benefits and Risks". National Academies Press (US). 7. Retrieved 28 March 2019.
- "Antibiotic Resistance". WHO. World Health Organization. Retrieved 28 March 2019.
- Dall, Chris (22 March 2018). "Price to Pay: Antibiotic-Resistant Infections Cost $2 billion a year". The Center for Infectious Disease Research and Policy.
- Yukhananov, Anna (11 April 2012). "U.S. seeks voluntary antibiotic limits in livestock". Reuters. Retrieved 28 August 2013.
- Bax, R.; Bywater, R.; Cornaglia, G.; Goossens, H.; Hunter, P.; Isham, V.; Jarlier, V.; Jones, R.; Phillips, I.; Sahm, D.; Senn, S.; Struelens, M.; Taylor, D.; White, A. (2001). "Surveillance of antimicrobial resistance-what, how and whither?". Clinical Microbiology and Infection. 7 (6): 316–325. doi:10.1046/j.1198-743x.2001.00239.x. PMID 11442565.
- U.S. Government Accountability Office (7 September 2011). Agencies Have Made Limited Progress Addressing Antibiotic Use in Animals (Report). Retrieved 27 August 2013.
- Mølbak, Kåre (1 December 2005). "Human health consequences of antimicrobial drug-resistant Salmonella and other foodborne pathogens". Clinical Infectious Diseases. 41 (11): 1613–1620. doi:10.1086/497599. ISSN 1537-6591. PMID 16267734.
- Mølbak, Kåre; Baggesen, Dorte Lau; Aarestrup, Frank Møller; Ebbesen, Jens Munk; Engberg, Jørgen; Frydendahl, Kai; Gerner-Smidt, Peter; Petersen, Andreas Munk; Wegener, Henrik C. (4 November 1999). "An Outbreak of Multidrug-Resistant, Quinolone-Resistant Salmonella enterica Serotype Typhimurium DT104". New England Journal of Medicine. 341 (19): 1420–1425. doi:10.1056/NEJM199911043411902. ISSN 0028-4793. PMID 10547404.
- Shea, Katherine M. (1 July 2003). "Antibiotic Resistance: What Is the Impact of Agricultural Uses of Antibiotics on Children's Health?". Pediatrics. 112 (Supplement 1): 253–258. ISSN 0031-4005. PMID 12837918.
- Center for Disease Control and Prevention (2013). Human Isolates Final Report (PDF). National Antibiotic Resistance Monitoring System: Enteric Bacteria (Report).
- Swartz, Morton N. (1 June 2002). "Human Diseases Caused by Foodborne Pathogens of Animal Origin". Clinical Infectious Diseases. 34 (Supplement_3): S111–S122. doi:10.1086/340248. ISSN 1058-4838. PMID 11988881.
- Bortolaia V; et al. (February 2016). "Human health risks associated with antimicrobial-resistant enterococci and Staphylococcus aureus on poultry meat". Clinical Microbiology and Infection. 22 (2): 130–40. doi:10.1016/j.cmi.2015.12.003. PMID 26706616.
- Wegener H (2003). "Antibiotics in animal feed and their role in resistance development". Current Opinion in Microbiology. 6 (5): 439–445. doi:10.1016/j.mib.2003.09.009. PMID 14572534.
- Wegener, Henrik C. (2012). "A15 Antibiotic Resistance—Linking Human and Animal Health". In Choffnes, E.R.; Relman, D.A.; Olsen, L.; Hutton, R.; Mack, A. (eds.). Antibiotic Resistance — Linking Human And Animal Health: Improving Food Safety Through a One Health Approach Workshop Summary. Washington DC: National Academies Press. doi:10.17226/13423. ISBN 978-0-309-25937-8. PMID 23230579. NBK114485.
- DeWaal, J.D.; Grooters, Susan Vaughn (May 2013). "Antibiotic Resistance in Foodborne Pathogens" (PDF). Center for Science in the Public Interest.
- Vieira, A. R.; Collignon, P.; Aarestrup, F. M.; McEwen, S. A.; Hendriksen, R. S.; Hald, T.; Wegener, H. C. (2011). "Association Between Antimicrobial Resistance inEscherichia coliIsolates from Food Animals and Blood Stream Isolates from Humans in Europe: An Ecological Study". Foodborne Pathogens and Disease. 8 (12): 1295–1301. doi:10.1089/fpd.2011.0950. PMID 21883007.
- Zhang, Sarah (2013). "Pig-manure fertilizer linked to human MRSA infections". Nature News. doi:10.1038/nature.2013.13752.
- Casey JA; et al. (2013). "High-Density Livestock Operations, Crop Field Application of Manure, and Risk of Community-Associated Methicillin-Resistant Staphylococcus aureus Infection in Pennsylvania". JAMA Internal Medicine. 173 (21): 1980–1990. doi:10.1001/jamainternmed.2013.10408. PMC 4372690. PMID 24043228.
- Chang, Qiuzhi; Wang, Weike; Regev‐Yochay, Gili; Lipsitch, Marc; Hanage, William P. (1 March 2015). "Antibiotics in agriculture and the risk to human health: how worried should we be?". Evolutionary Applications. 8 (3): 240–247. doi:10.1111/eva.12185. ISSN 1752-4571. PMC 4380918. PMID 25861382.
- Hurd, H. Scott; Doores, Stephanie; Hayes, Dermot; Mathew, Alan; Maurer, John; Silley, Peter; Singer, Randall S.; Jones, Ronald N. (1 May 2004). "Public Health Consequences of Macrolide Use in Food Animals: A Deterministic Risk Assessment". Journal of Food Protection. 67 (5): 980–992. doi:10.4315/0362-028X-67.5.980. ISSN 0362-028X. PMID 15151237.
- Hurd, H. Scott; Malladi, Sasidhar (June 2008). "A stochastic assessment of the public health risks of the use of macrolide antibiotics in food animals" (PDF). Risk Analysis. 28 (3): 695–710. doi:10.1111/j.1539-6924.2008.01054.x. ISSN 1539-6924. PMID 18643826.
- Allen H. K.; Trachsel J.; Looft T.; Casey T. A. (2014). "Finding alternatives to antibiotics". Annals of the New York Academy of Sciences. 1323 (1): 91–100. Bibcode:2014NYASA1323...91A. doi:10.1111/nyas.12468. PMID 24953233.
- Hume M. E. (2011). "Historic perspective: Prebiotics, probiotics, and other alternatives to antibiotics". Poultry Science. 90 (11): 2663–9. doi:10.3382/ps.2010-01030. PMID 22010256.
- Griggs J. P.; Jacob J. P. (2005). "Alternatives to Antibiotics for Organic Poultry Production" (PDF). Poultry Science. 14 (4): 750–756. doi:10.1093/japr/14.4.750.
- Doyle, M.E. 2001: Alternatives to Antibiotic Use for Growth Promotion in Animal Husbandry. Food Research Institute, University of Wisconsin-Madison.
- Joerger R.D. (2003). "Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages". Poultry Science. 82 (4): 640–647. doi:10.1093/ps/82.4.640. PMID 12710486.
External links
- PBS report on antibiotics in livestock production
- Fix Food, Fix Antibiotics, a 90-second video explaining the problem of antibiotic resistance and campaigning for action
- Pew Trust campaign for restricting antibiotic use
- Antibiotic Resistance and the Use of Antibiotics in Animal Agriculture: Hearing before the Subcommittee on Health of the Committee on Energy and Commerce, House of Representatives, One Hundred Eleventh Congress, Second Session, July 14, 2010
- Resources on antibiotic use and resistance