We need you! Join our contributor community and become a WikEM editor through our open and transparent promotion process.
Kaposi's sarcoma
From WikEM
Contents
Background
- Different classifications of Kaposi’s sarcoma based on patient population
- Associated with HHV-8
- Most common cancer in HIV/AIDS patients
- Kaposi’s Sarcoma can be unmasked or induced by initiation of steroids or HAART
- Cutaneous lower extremity lesions most common presentation
- Kaposi’s sarcoma is an AIDS-defining illness
- Treat with HAART and/or chemo
History
- First described by Hungarian Dr. Moritz Kaposi in 1872; rare until 1980s with emergence of HIV/AIDS (1981 cluster of Kaposi’s sarcoma in young men in NYC and California; 1982 listed as AIDS-defining illness by CDC)
- Human Herpesvirus 8 (HHV8)
- Large, double-stranded DNA virus
- Necessary but NOT sufficient to cause disease
- Also associated with body-cavity based lymphomas, multicentric Castleman’s disease (angiofollicular lymph node hyperplasia)
Classifications
- Classic Kaposi’s (CK)
- Indolent, chronic form, rarely disseminated
- Skin most commonly effected, lower extremities
- ~3:1 Male:Female, Age > 60 years old with Mediterranean, Eastern European or Middle Eastern origin
- Endemic Kaposi’s sarcoma (Africa)
- Male adults or children of both sexes
- Equatorial/Sub-Saharan Africa
- Aggressive form with lymph node involvement (lymphedema)
- 27%, 35%, and 24% of total cancer burden in Uganda, Zimbabwe and Mozambique, respectively
- AIDS-KS
- Developed countries: Men who have sex with men
- Developing countries: Heterosexual men and women (Africa)
- Aggressive disease – most commonly in lungs, gastrointestinal tract, mouth, and genitalia
- Most common cancer in HIV/AIDS patients
- Iatrogenic due to immunosuppression – Most commonly organ transplant-associated
- Increased risk after solid organ transplantation
- Transplant itself may transmit HHV-8 infection
- Male > Female (3.3:1), average age 43 yo
- Cutaneous lesion presentation most common
- More common in liver than kidney transplant patients
- Immune Reconstitution Inflammatory Syndrome (IRIS) Phenomenon
- Initiation of retroviral therapy can cause the development of KS
- True unmasked KS-IRIS begins within 6 months of initiating therapy, and the rates remain unclear
Clinical Features
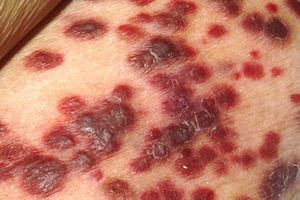
- Cutaneous
- Heterogeneous appearance
- Color – dark blue, reddish, purple, violaceous, dark brown/black
- Location – extremities, most commonly the feet
- Lesions: macules, plaques, nodules
- Associated lymphedema
- Pulmonary
- Gradual Onset (2-4 weeks)
- Cough
- Dyspnea
- Fever
- Hemoptysis
- Asymptomatic
- Gastrointestinal
- Bleeding
- Obstruction
Differential Diagnosis
- Cutaneous Lesions:
- Bacillary angiomatosis
- Purpura
- Hematomas
- Angiomas
- Dermatofibromas
- Nevi
- Pulmonary Lesions in HIV/AIDs:
- Bacterial Pneumonia
- PCP
- TB
- Fungal Infections
- Anorectal Lesions in HIV/AIDs:
- Common: Anal fissure, Abscess and/or fistula, Hemorrhoid, Pilonidal disease
- STDs: Gonorrhea, Chlamydial infection, Herpes, Chancroid, Syphilis, Condyloma acuminatum
- Infectious: TB, CMV infection, actinomycosis, cryptococcosis
- Neoplastic: Lymphoma, Kaposi’s sarcoma, squamous cell carcinoma
HIV associated conditions
- HIV neurologic complications
- HIV pulmonary complications
- Ophthalmologic complications
- Other
- HAART medication side effects[1]
- Lactic acidosis
- Neuropyschiatric effects
- Hepatic toxicity
- Renal toxicity
- Steven-Johnson's
- Cytopenias
- GI symptoms
- Endocrine abnormalities
Evaluation
- Clinical impression
- Biopsy is gold standard for definitive diagnosis
- WHO Clinical Staging of HIV/AIDS
- Stage 4: Presumptive Diagnosis of HIV/AIDS can be made from clinical signs of Kaposi’s
- GI manifestations
- Unlikely visible on XR; can be seen on flex sig or colonoscopy
- Pulmonary Manifestations
- Heterogeneous on XR - nodular, interstitial and/or alveolar infiltrates, pleural effusion, hilar and/or mediastinal adenopathy, or an isolated nodule
Staging
- Classic Kaposi's Sarcoma
- No staging system. It is excluded in TNM Staging of soft tissue sarcomas by American Joint Committee on Cancer (AJCC)
- AIDS-KS Staging
| Good Risk (all of the following) | Poor Risk (any of the following) | |
| Tumor, T | T0: Skin and/or lymph nodes and/or minimal oral disease | TI: Tumor-associated edema or ulceration, extensive oral KS, GI KS, KS in other non-nodal viscera |
| Immune System, I | CD4 > 200 | CD4 < 200 |
| Systemic Illness, S | S0: No history of OI or thrust, No "B" Symptoms (fever, night sweats, weight loss, diarrhea > 2 weeks) Karnofsky performance > 70 | SI: History of OI and/or thrush, "B" symptoms, Karnofsky performance <70, Other HIV-related illness |
Management
- Highly Active Anti-retroviral therapy (HAART):
- Can achieve remission in 60-90% remission in stage T0 disease
- Can combine with chemotherapy
- Chemotherapy:
- First-line: Pegylated liposomal doxorubicin (PLD) 20mg/m2 every three weeks (unless cardiac contraindication)
- Second-line: Vinblastine, Bleomycin, Paclitaxel, Etoposide, Gemcitabine
- Cutaneous Specific Treatment:
- Alitretinoin (9-cis-retinoic acid) topical gel 0.1% FDA-approved for cutaneous AIDS-associated KS
- Intralesion Injection: Vinblastine or Bleomycin or INF-alpha
- Vinblastine 0.2 – 0.3mg/mL solution with a volume of 1 mL per 0.5 cm2 of lesion
- IFNa - FDA approved for AIDS-associated KS
- Radiation Therapy
- 30 Gy in 15 daily 2 Gy fractions
- Other Drug Trials:
- Bevacizumab - an anti-VEGF-A
- Imatinib - monoclonal antibody tyrosine kinase receptor
- Steroids
- Associated with generation and exacerbation of KS
- Important implications because of the high use of steroids in this patient population (HIV/AIDS, transplant)
- Once steroids are removed, KS can regress
See Also
External Links
References
- Farge, D. "Kaposi's Sarcoma in Organ Transplant Recipients. The Collaborative Transplantation Research Group of Ile De France." The European Journal of Medicine 2.6 (1993): 339-43.
- Fenig, Eyal, Baruch Brenner, Erica Rakowsky, Moshe Lapidoth, Alan Katz, and Aaron Sulkes. "Classic Kaposi Sarcoma." American Journal of Clinical Oncology 21.5 (1998): 498-500.
- Krown, SE, C. Metroka, and JC Wemz. "Kaposi's Sarcoma in the Acquired Immune Deficiency Syndrome: A Proposal for Uniform Evaluation, Response and Staging Criteria." Journal of Clinical Oncology 7.9 (1989): 1201-207.
- Krown, SE, MA Testa, and J. Huang. "AIDS-related Kaposi's Sarcoma: Prospective Validation of the AIDS Clinical Trials Group Staging Classification. AIDS Clinical Trials Group Oncology Committee." Journal of Clinical Oncology 15.9 (1997): 3085-092.
- Moosa, M.r. "Kaposi's Sarcoma in Kidney Transplant Recipients: A 23-year Experience." QJM 98.3 (2005): 205-14.
- Osawa, R., N. Kato, T. Yanagi, and N. Yamane. "Clearance of Recurrent, Classical Kaposi’s Sarcoma Using Multiple Paclitaxel Treatments." Acta Derm Venereol Acta Dermato-Venereologica 87.5 (2007): 435-36. Web.
- Robey, Rebecca C., and Mark Bower. "Facing up to the Ongoing Challenge of Kaposiʼs Sarcoma." Current Opinion in Infectious Diseases 28.31 (2015).
- Rosen, Peter, and John A. Marx. Rosen's Emergency Medicine: Concepts and Clinical Practice. 8th ed. Philadelphia: Elsevier Saunders, 2014.
- Safai, B., and R. A. Good. "Kaposi's Sarcoma: A Review and Recent Developments." CA: A Cancer Journal for Clinicians 31.1 (1981): 2-12.
Cite error: <ref> tags exist, but no <references/> tag was found
Authors
Natalie Sciano, Ross Donaldson, Daniel Ostermayer, Neil Young