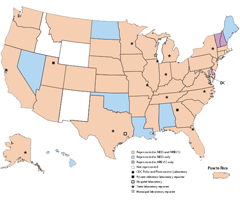
The National Enterovirus Surveillance System (NESS) is a passive, voluntary surveillance system that monitors laboratory detections of enteroviruses and human parechoviruses in the United States. Participating laboratories are encouraged to report basic data, including serotype, on specimens. Since the 1960s, public health practitioners, researchers, and clinicians have used NESS data to determine patterns in the circulation of individual enterovirus and parechovirus types.
Most Common Enteroviruses Reported in the U.S., 2009-2013
| Enterovirus | Number of Positive Specimens |
|---|---|
| 1 Coxsackievirus A6 | 12.3% of specimens |
| 2 Human Parechovirus 3 | 12.3% of specimens |
| 3 Echovirus 11 | 7.9% of specimens |
| 4 Echovirus 18 | 5.6% of specimens |
| 5 Coxsackievirus A9 | 5.1% of specimens |
| 6 Coxsackievirus B4 | 5% of specimens |
| 7 Echovirus 30 | 5% of specimens |
For more information about the most recent enterovirus data, see CDC MMWR Enterovirus and Human Parechovirus Surveillance – United States, 2009 – 2013.
Data Collection
 NESS accepts electronic reports of enterovirus and parechovirus typing results. If your laboratory is interested in participating, please contact us.
NESS accepts electronic reports of enterovirus and parechovirus typing results. If your laboratory is interested in participating, please contact us.
- Page last reviewed: March 11, 2016
- Page last updated: March 11, 2016
- Content source:


 ShareCompartir
ShareCompartir