Sexual Assault | Questions & Answers | 2015 STD Treatment Guidelines
Question: What is the rationale for administrating HPV vaccine in the case of sexual assault?
Victims of sexual assault are at increased risk for STI acquisition, including HPV, since they are at increased risk for repeat sexual assault, increased number of lifetime sex partners, lower odds of being screened for cervical cancer, and increased risk of abnormal cervical cytology. Although the HPV vaccine is not curative and does not prevent infection in these cases, it provides a measure of prevention for HPV complications, given the increased risk from current and future exposure. Survivors of sexual assault are also at higher risk of being lost to follow-up. It is therefore recommended that HPV vaccine be offered and administered during the initial examination.
HPV vaccination is recommended for female survivors aged 9–26 years and male survivors aged 9–21 years. For MSM who have not received HPV vaccine, or who have been incompletely vaccinated, vaccine can be administered through age 26 years. The vaccine should be administered to sexual assault survivors at the time of the initial examination, and the follow-up doses administered at one to two months and then six months after the first dose. Efforts should be made to connect survivors to care systems that will provide clinical continuity, mental health support, and monitor adherence.
Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Recomm Rep 2015;64(No. RR-3): 1–137.
Farley M, Golding, J Minkoff, J. (2002). Is a history of trauma associated with a reduced likelihood of cervical cancer screening? The Journal of Family Practice, 51,827–831.
Sadler A, Mengeling M, Syrop C, Torner J, Booth B. (2011).Military service, life span sexual assault exposures, and cervical cytologic abnormalities. Journal of Women’s Health, 1–4 doi:10.1089/jwh.2010.2399.
Fontenot HB. (2013). Intersection of HPV and Sexual Assault: An Opportunity for Practice Change. Journal of Forensic Nursing. Vol 9, No 3. 146–154.
Question: What is the rationale for testing for STDs and providing prophylactic treatment? What is the recommended timeline for STD testing after sexual assault?
The decision to obtain specimens for STD diagnosis should be made on an individual basis. If the decision is made to screen for STDs, this should be done during initial exam if possible. However, this is not necessarily for the purposes of legal or forensic evidence as, depending on previous sexual history and experiences, a diagnosis of an STD may not be the result of an assault. On average, the incubation period for chlamydia and gonorrhea can be two to six days, and for syphilis about three weeks, but can range from nine days to three months. All 50 states limit the use of the survivor’s previous history of an STD diagnosis and sexual history as evidence in the court of law.
Given that compliance with follow-up is generally suboptimal, it may be beneficial to provide prophylactic or empirical treatment for STDs. If testing prior to treatment suggests exposure to an STD, appropriate enhancement of follow-up efforts are indicated to document clearance of infection and provision of additional evaluation and supportive services.
Excerpt from the 2015 CDC STD treatment guidelines:
“If initial tests are negative and treatment was not provided, examination for STDs can be repeated within 1–2 weeks of the assault; repeat testing detects infectious organisms that might not have reached sufficient concentrations to produce positive test results at the time of initial examination. For survivors who are treated during the initial visit, regardless of whether testing was performed, post-treatment testing should be conducted only if the survivor reports having symptoms. A follow-up examination at 1–2 months should also be considered to reevaluate for development of anogenital warts, especially among sexual assault survivors who received a diagnosis of other STDs. If initial test results were negative and infection in the assailant cannot be ruled out, serologic tests for syphilis can be repeated at 4–6 weeks and 3 months; HIV testing can be repeated at 6 weeks and at 3 and 6 months using methods to identify acute HIV infection”
Recommendations for postexposure HIV risk assessment of adolescent and adult survivors within 72 hours of sexual assault:
-
- Assess risk for HIV infection in the assailant, and test that person for HIV whenever possible.
- Use the algorithm to evaluate the survivor for the need for HIV nPEP (Figure 1).
- Consult with a specialist in HIV treatment if nPEP is being considered.
- If the survivor appears to be at risk for acquiring HIV from the assault, discuss nPEP, including benefits and risks.
- If the survivor chooses to start nPEP (312), provide enough medication to last until the follow-up visit at three to seven days after initial assessment and assess tolerance to medications.
- If nPEP is started, perform CBC and serum chemistry at baseline.
- Perform an HIV antibody test at original assessment; repeat at six weeks, three months, and six months.
Assistance with nPEP-related decisions can be obtained by calling the National Clinician’s Post Exposure Prophylaxis Hotline (PEP Line) (telephone: (888) 448–4911).
If the client declines nPEP, and the initial HIV test is negative, it should be repeated within two to four weeks with a fourth generation Ag/Ab assay.
FIGURE 1. Algorithm for evaluation and treatment of possible nonoccupational HIV exposures
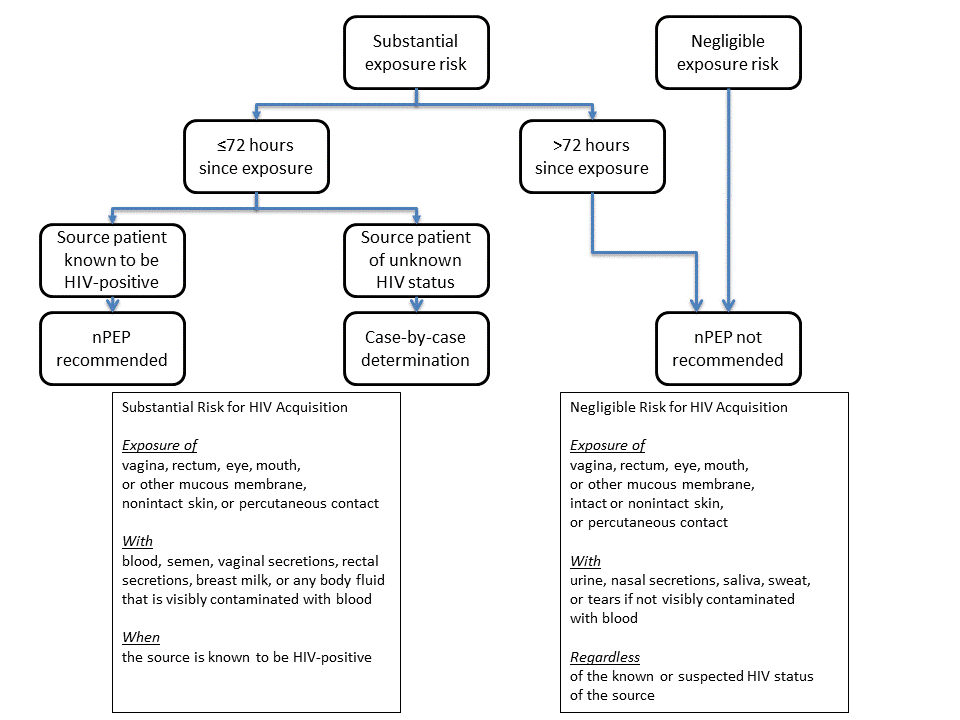
Source: CDC. Antiretroviral post exposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States. MMWR Recomm Rep 2005;54(No. RR-02):1–20.
Reference:
Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Recomm Rep 2015;64(No. RR-3): 1–137.
- Page last reviewed: February 11, 2016
- Page last updated: February 10, 2016
- Content source:


 ShareCompartir
ShareCompartir