Key Findings—A Closer Look at the Link Between Specific SSRIs and Birth Defects
Share with your friends
Important Reminder for Women
Depression and other mental health conditions can be serious. Many women need to take medication during pregnancy to appropriately manage their symptoms. If you are pregnant or thinking about becoming pregnant, talk with your doctor about any medications you are taking or thinking about taking.
Abruptly stopping the use of medicines to treat these conditions can have serious consequences. Women should not change medications or stop taking medications without first talking with their doctor about available options.
New CDC study findings refute some earlier reported links but confirm other links observed between birth defects and some selective serotonin reuptake inhibitors (SSRIs), which are medications used to treat depression and other mental health conditions. Researchers found that some birth defects occur about two or three times more frequently among babies born to women who took certain types of SSRI medications early in pregnancy. This analysis can help guide healthcare providers and women to the safest options to appropriately treat depression or other mental health conditions during pregnancy while minimizing the risk of major birth defects in the developing baby. You can read the article’s abstract here, and read more below for a summary of this study’s findings.
What did we already know?
Selective serotonin reuptake inhibitors (SSRIs) are medications used to treat depression and other mental health conditions. Previous studies provide conflicting evidence about potential links between the use of SSRIs during pregnancy and certain birth defects.
What does this study add?
In this CDC study published in The BMJ, researchers re-assessed several previously reported links between SSRI use and birth defects using more recent data. These results reflect not only the new data, but also incorporate results from previously published independent studies. Researchers found some birth defects occur about two or three times more frequently among babies born to women who took certain SSRI medications, like fluoxetine and paroxetine, early in pregnancy. However, links between birth defects and other SSRIs, like sertraline, were not observed in this CDC study.
What were this study’s main findings?
- Researchers investigated links reported in previous studies by combining those results with new data.
- In previous studies, fluoxetine appeared to be linked with four types of birth defects. In this study, researchers observed fluoxetine to be linked with these two birth defects:
- Heart defects with obstruction of the right ventricular outflow tract
- Craniosynostosis, a birth defect of a baby’s skull
- Researchers still observed five out of the seven previous links between paroxetine and certain birth defects. In this study, paroxetine appeared to be linked with these birth defects:
- Anencephaly, a birth defect of a baby’s brain and skull
- Atrial septal defects, a type of heart defect
- Heart defects with obstruction of the right ventricular outflow tract
- Gastroschisis, a birth defect of the abdominal (belly) wall
- Omphalocele, another type of birth defect of the abdominal (belly) wall
- Reassuringly, researchers did not confirm links between sertraline, the SSRI used most often, and any of the birth defects observed in previous studies.
- In total, 7 of the 21 previously reported specific SSRI-birth defect links were confirmed in this analysis.
- In previous studies, fluoxetine appeared to be linked with four types of birth defects. In this study, researchers observed fluoxetine to be linked with these two birth defects:
- Despite the increased risks for certain birth defects from some SSRIs found in this study, the actual risk for a birth defect among babies born to women taking one of these medications is still very low. Because these specific types of birth defects are rare, even doubling the risk still results in a low absolute risk. For example, the risks for heart defects with obstruction of the right ventricular outflow tract could increase from 10 per 10,000 births to about 24 per 10,000 births among babies of women who are treated with paroxetine early in pregnancy.
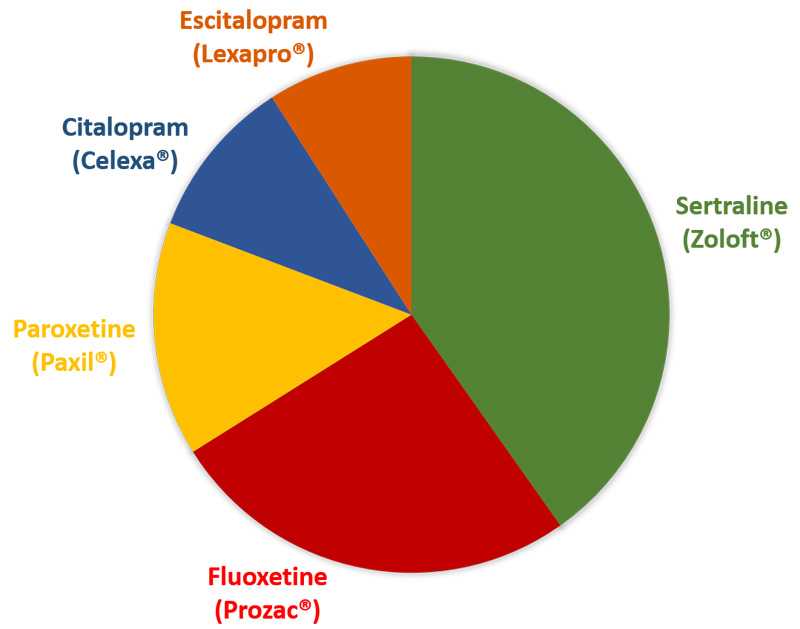
Distribution of specific SSRIs used among women whose baby did not have a birth defect
About this Study
- For this study, researchers used new data to look at previous reported links between use of specific SSRI medications just before or during early pregnancy and the occurrence of certain birth defects. Researchers looked at links with five different SSRI medications: citalopram, escitalopram, fluoxetine, paroxetine, and sertraline.
- The study used data from the National Birth Defects Prevention Study (NBDPS), one of the largest studies aimed at understanding factors that increase the risk for major birth defects in the United States.
- Researchers used a Bayesian approach, meaning they used findings from previous studies to inform their new analyses using NBDPS data from 1997-2009. In these analyses, researchers accounted for a mother’s race/ethnicity, education, smoking, and obesity prior to pregnancy, since these factors can also affect the risk for certain birth defects.
- Researchers only assessed those links that had previously been reported in other studies of SSRI medication use and specific birth defects. Future studies will address additional health outcomes.

Medication Use during Pregnancy: CDC’s Activities
CDC’s National Center on Birth Defects and Developmental Disabilities (NCBDDD) is working to improve the health of women and babies through its Treating for Two: Safer Medication Use in Pregnancy initiative. Through Treating for Two, CDC is working with its partners, other federal agencies, and the public to understand trends in medication use among pregnant women and women of reproductive age, and to provide women and healthcare providers with information about the safety or risk of using specific medications during pregnancy. This information will allow women and their doctors to make informed decisions about treating health conditions during pregnancy.
Treating for Two focuses on the following activities:
- Better research: Treating for Two is working to expand research on medication use and pregnancy outcomes.
- Reliable guidance: Treating for Two is building the foundation to establish a process to review evidence and develop guidance for treating conditions in pregnancy.
- Informed decisions: Through these activities, Treating for Two will provide credible and reliable information to healthcare providers and the public to support treatment decisions in pregnancy.
For more information, visit www.cdc.gov/treatingfortwo
Reference
Reefhuis J, Devine O, Friedman JM, Louik C, Honein MA, and the National Birth Defects Prevention Study. Specific SSRIs and birth defects: bayesian analysis to interpret new data in the context of previous reports. BMJ. 2015. [epub ahead of print].
- Page last reviewed: July 8, 2015
- Page last updated: July 8, 2015
- Content source:


 ShareCompartir
ShareCompartir
